A method for preparing silver clusters using hydrogen as a reducing agent
A reducing agent, hydrogen technology, used in the synthesis of noble metal nanomaterials, can solve the problems of limiting the wide application of AgNCs, toxic side effects, poor stability, etc., and achieve excellent fluorescence characteristics and stability, large Stokes shift, and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
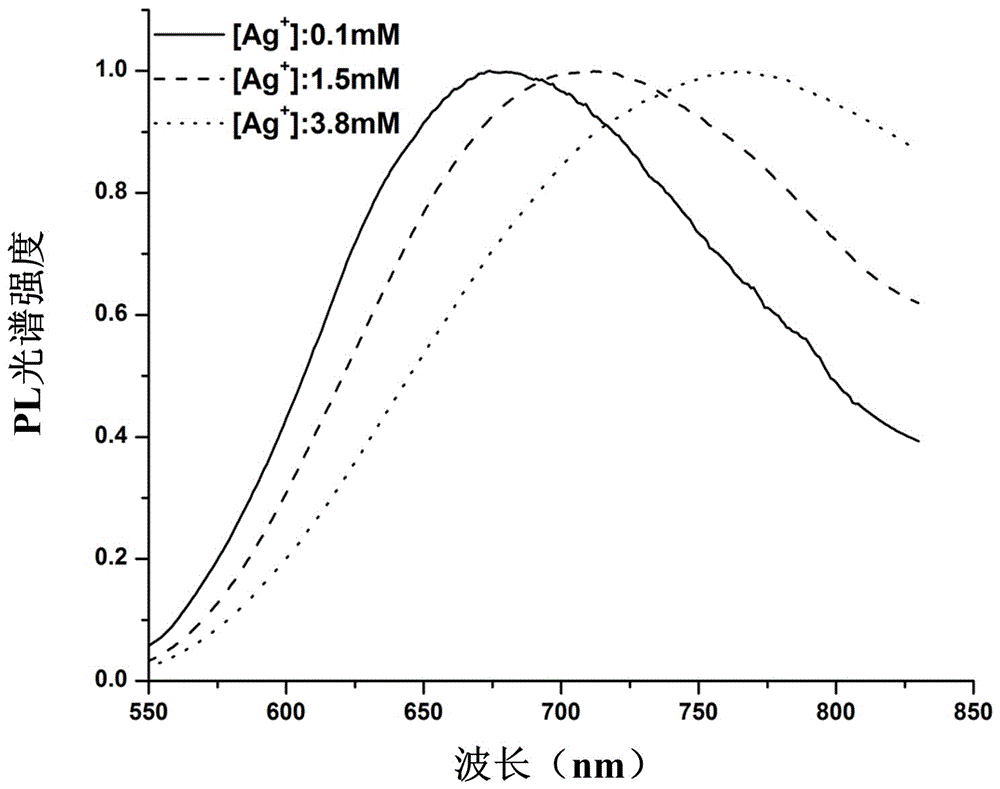
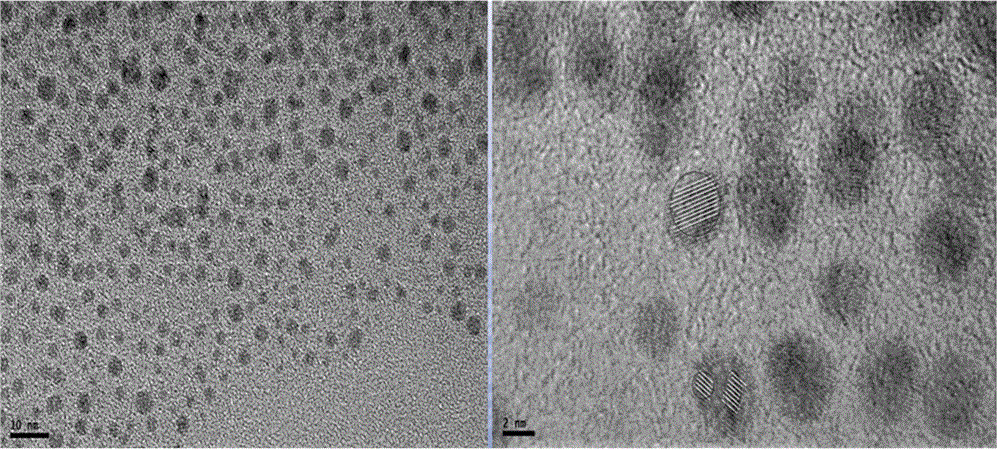
[0024] In this embodiment, using polyacrylic acid as a template and hydrogen as a reducing agent, the polyacrylic acid and silver nitrate were reacted at 25° C. for 5 to 40 minutes under the hydrogen atmosphere, thereby obtaining silver clusters with near-infrared emission; Wherein, the molar ratio of silver ions in the silver nitrate to the polyacrylic acid monomer is 1:6. The specific steps are described below.
[0025] Use a pipette to accurately pipette 5ml of deionized water into a 10ml beaker, and add 6μl of freshly prepared 0.5 mol / l PAA solution and 1μl of 0.5 mol / l AgNO with a pipette gun while stirring 3 solution to obtain a mixed solution, after calculation: Ag in the mixed solution + The concentration is 0.1mmol / l, Ag + The molar ratio to PAA monomer is 1:6. The mixed solution was stirred under dark conditions for 10 minutes, then it was transferred to a 50ml autoclave, and filled with 1.5bar high-purity H 2 , and stirred at 25°C for 5-40 minutes. Finally, the...
Embodiment 2
[0028] In this embodiment, using polyacrylic acid as a template and hydrogen as a reducing agent, the polyacrylic acid and silver nitrate were reacted at 25° C. for 5 to 40 minutes under the hydrogen atmosphere, thereby obtaining silver clusters with near-infrared emission; Wherein, the molar ratio of silver ions in the silver nitrate to the polyacrylic acid monomer is 1:6. The specific steps are described below.
[0029] Use a pipette to accurately pipette 5ml of deionized water into a 10ml beaker, add 90μl of freshly prepared 0.5mol / l PAA solution, 15μl of 0.5mol / l AgNO 3 solution to obtain a mixed solution, after calculation: Ag in the mixed solution + Concentration of 1.5mmol / l, Ag + The molar ratio to PAA monomer is 1:6. The mixed solution was stirred under dark conditions for 10 minutes, then it was transferred to a 50ml autoclave, and filled with 1.5bar high-purity H 2 , and stirred at 25°C for 5-40 minutes. Finally, the prepared AgNCs solution was stored in a refr...
Embodiment 3
[0032] In this embodiment, using polyacrylic acid as a template and hydrogen as a reducing agent, the polyacrylic acid and silver nitrate were reacted at 25° C. for 5 to 40 minutes under the hydrogen atmosphere, thereby obtaining silver clusters with near-infrared emission; Wherein, the molar ratio of silver ions in the silver nitrate to the polyacrylic acid monomer is 1:6. The specific steps are described below.
[0033]Use a pipette to accurately pipette 5ml of deionized water into a 10ml beaker, and add 240μl of freshly prepared 0.5 mmol / l PAA solution and 40μl of 0.5 mmol / l AgNO 3 solution to obtain a mixed solution, after calculation: Ag in the mixed solution + The concentration is 3.8mmol / l, Ag + The molar ratio to PAA monomer is 1:6. The mixed solution was stirred under dark conditions for 10 minutes, then it was transferred to a 50ml autoclave, and filled with 1.5bar high-purity H 2 , and stirred at 25°C for 5-40 minutes. Finally, the prepared AgNCs solution was s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com