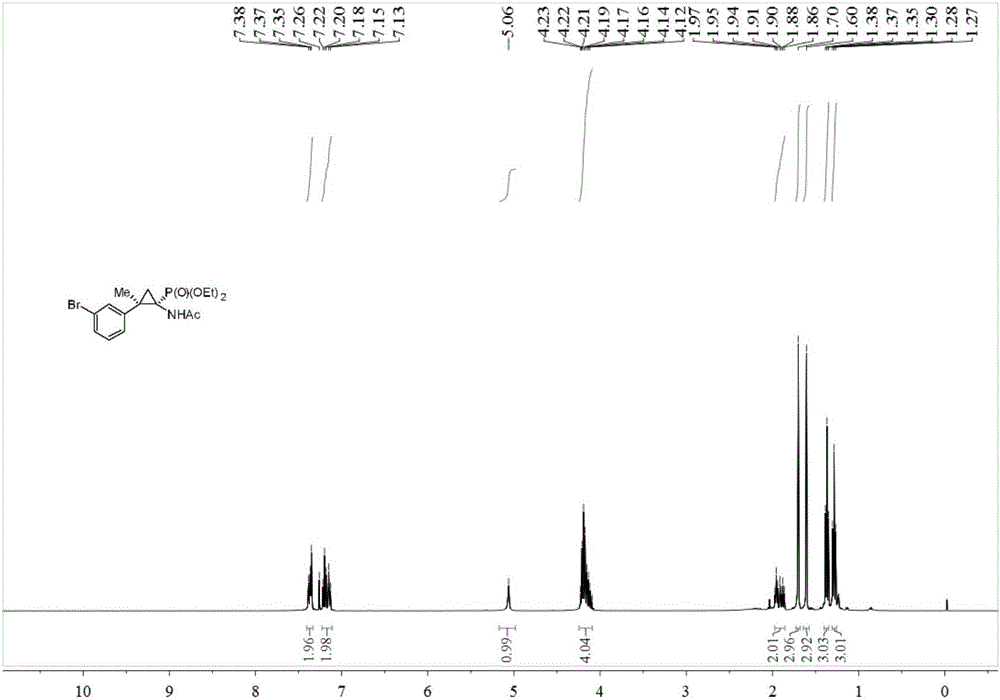
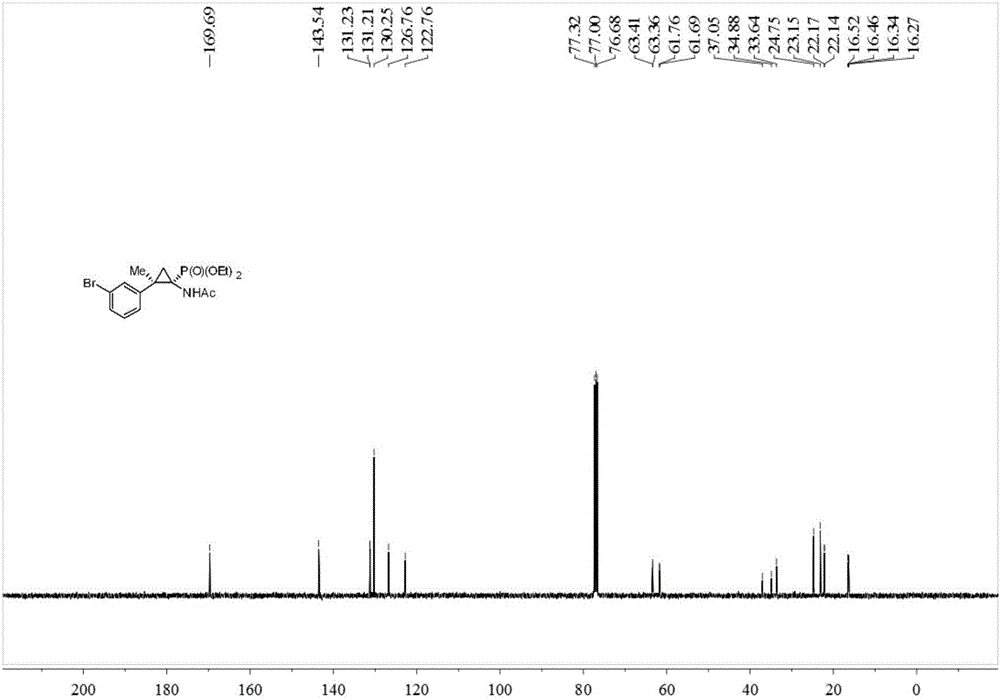
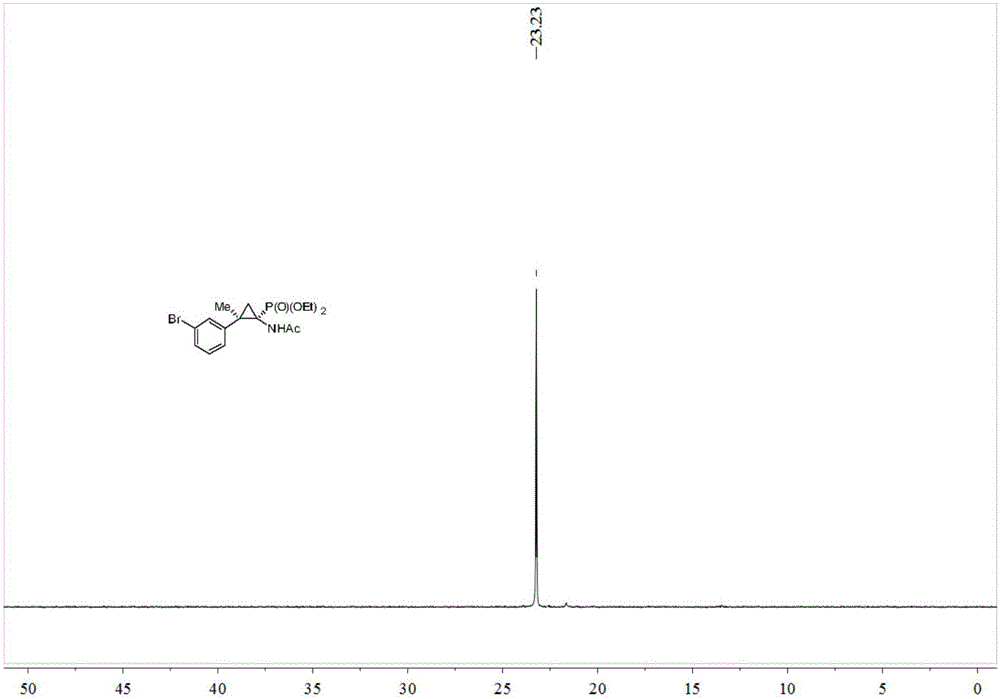
Synthesis method of cyclopropane phosphoramidate compound comprising continuous quaternary carbon center
A technology of cyclopropane phosphoramidate and acetamido enphosphate, applied in the field of synthesis of cyclopropane phosphoramidate compounds containing continuous quaternary carbon centers, achieving high atom economy, wide substrate adaptability, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 0.2 mmol of acetophenone-p-toluenesulfonylhydrazone, 0.2 mmol of potassium tert-butoxide, 0.02 mmol of benzyltriethylammonium chloride (TEBAC) and 2 ml of toluene into the reaction tube, under inert atmosphere Stir and react at room temperature at 600 rpm under protection for 2 hours, then stop stirring. Add 0.1 mmol of acetaminophenophosphate (Nicolas Lefevre, Jean-Louis Brayer, Benoit Folleas, Sylvain Darses, Organic Letters, 2013, 15, 4274–4276), stir and react at 90 degrees Celsius at 600 rpm for 12 hours, then stop heating And stir, cool to room temperature. Add 4 mL of water, extract 3 times with ethyl acetate, combine the organic phases and use 0.5 g of anhydrous magnesium sulfate to dry, filter, evaporate the solvent under reduced pressure, and then separate and purify by column chromatography to obtain the target product. The eluent was petroleum ether:ethyl acetate mixed solvent with a volume ratio of 10:1, and the yield was 62%.
Embodiment 2
[0035]Add 0.2 mmol of acetophenone p-toluenesulfonylhydrazone, 0.2 mmol of sodium carbonate, 0.02 mmol of TEBAC and 2 ml of toluene into the reaction tube, stir and react at room temperature at 600 rpm for 2 hours under the protection of an inert atmosphere, and stop stirring. Add 0.1 mmol of acetaminophenophosphate (Nicolas Lefevre, Jean-Louis Brayer, Benoit Folleas, Sylvain Darses, Organic Letters, 2013, 15, 4274–4276), stir the reaction at 90 degrees Celsius at 600 rpm for 12 hours, then stop Heat and stir, and cool to room temperature. Add 4 mL of water, extract 3 times with ethyl acetate, combine the organic phases and use 0.5 g of anhydrous magnesium sulfate to dry, filter, evaporate the solvent under reduced pressure, and then separate and purify by column chromatography to obtain the target product. The eluent was petroleum ether:ethyl acetate mixed solvent with a volume ratio of 10:1, and the yield was 72%.
Embodiment 3
[0037] Add 0.2 mmol of acetophenone p-toluenesulfonylhydrazone, 0.2 mmol of cesium carbonate, 0.02 mmol of TEBAC and 2 ml of toluene into the reaction tube, stir and react at room temperature at 600 rpm for 2 hours under the protection of an inert atmosphere, and stop stirring. Add 0.1 mmol of acetaminophenophosphate (Nicolas Lefevre, Jean-Louis Brayer, Benoit Folleas, Sylvain Darses, Organic Letters, 2013, 15, 4274–4276), stir the reaction at 90 degrees Celsius at 600 rpm for 12 hours, then stop Heat and stir, and cool to room temperature. Add 4 mL of water, extract 3 times with ethyl acetate, combine the organic phases and use 0.5 g of anhydrous magnesium sulfate to dry, filter, evaporate the solvent under reduced pressure, and then separate and purify by column chromatography to obtain the target product. The eluent was petroleum ether:ethyl acetate mixed solvent with a volume ratio of 10:1, and the yield was 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com