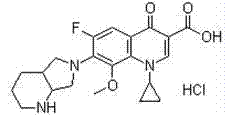
Preparation method of moxifloxacin hydrochloride
A technology of moxifloxacin hydrochloride and carboxylic acid, which is applied in the field of preparation of moxifloxacin hydrochloride, can solve the problems of high cost, high impurity content of moxifloxacin hydrochloride, expensive reagents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Dissolve 248.6g of ethyl borate chelate and 81.6g (S,S)-2,8-diazabicyclo[4.3.0]nonane (molar ratio 1:1.1) in 994.4mL acetonitrile (248.6* 4) React with 90.2mL triethylamine (molar ratio 1:1.1) at 60°C, check the progress of the reaction by TLC, and determine the end of the reaction. After the reaction is complete, cool to 25°C, add hydrochloric acid, adjust the pH to 6, stir and crystallize for 20 minutes, slowly add hydrochloric acid dropwise to adjust the pH to 2. Cool to 15°C, stir and crystallize for 1 hour. Filter with suction, drain, and wash with cold ethanol. The product was dried to obtain 237.3g, the yield was 92.2%, and the total impurities were 2.5‰.
Embodiment 2
[0057] 497.2g of ethyl borate chelate and 163.1g (S,S)-2,8-diazabicyclo[4.3.0]nonane (molar ratio 1:1.1) were dissolved in 1988.8mL acetonitrile (497.2* 4) React with 180.4ml triethylamine (molar ratio 1:1.1) at 65°C, check the progress of the reaction by TLC, and determine the end of the reaction. After the reaction is complete, cool to 35°C, add hydrochloric acid, adjust the pH to 4, stir and crystallize for 30 minutes, slowly add hydrochloric acid dropwise to adjust the pH to 0.5. The temperature was lowered to 10°C, and the mixture was stirred and crystallized for 3 hours. Filter with suction, drain, and wash with cold ethanol. The dried product is 489.5 g, the yield is 95.1%, and the total impurities are 2.7‰.
Embodiment 3
[0059] Dissolve 248.6g of ethyl borate chelate and 81.6g (S,S)-2,8-diazabicyclo[4.3.0]nonane (molar ratio 1:1.1) in 994.4mL acetonitrile (248.6* 4) React with 90.2mL triethylamine (molar ratio 1:1.1) at 62°C, check the progress of the reaction by TLC, and determine the end of the reaction. After the reaction is complete, cool to 30°C, add hydrochloric acid, adjust the pH to 5, stir and crystallize for 25 minutes, slowly add hydrochloric acid dropwise to adjust the pH to 1. The temperature was reduced to 12°C, and the mixture was stirred and crystallized for 2 hours. Filter with suction, drain, and wash with cold ethanol. The dried product was 241.4g, the yield was 93.8%, and the total impurities were 2.3‰.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com