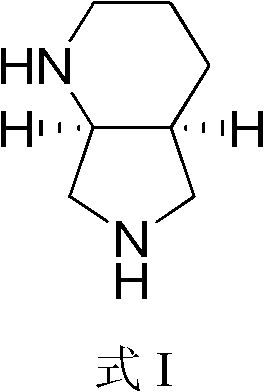
Method for chiral synthesis of (S,S)-2-8-diazabicyclononane
A technology of chiral synthesis and chiral separation, applied in the direction of organic chemistry, etc., can solve the problems of long reaction route, low production efficiency, late separation steps, etc., to save raw materials and reagents, improve efficiency, and reduce costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1 (S, S)-2, the preparation of 8-diazabicyclo [4.3.0] nonane (formula I)
[0049] 1.1 Hydrogenation reaction
[0050] Dissolve 100g of methyl 2,3-pyridinedicarboxylate in 500mL of toluene, then transfer it into an autoclave, add 5g of 10% palladium carbon, the system is closed, and after three times of hydrogen replacement, keep the stirring speed at 500 rpm and the system pressure React at 1 MPa and 50-60°C for 5 hours, lower the temperature to release excess hydrogen, filter the reaction solution, and distill the filtrate to remove toluene to obtain 101 g of almost colorless liquid with a yield of 98%.
[0051] The product was identified as cis-2,3-piperidine-dicarboxylic acid methyl ester by NMR, and the NMR data was:
[0052] 1 H NMR (500MHz, CDCl 3 ): δ1.48-1.53(m, 2H), 1.77-1.82(m, 1H), 2.14-2.18(m, 1H), 2.26(br, 1H), 2.67-2.72(m, 1H), 2.99-3.07( m, 2H), 3.65 (d, J = 3.5 Hz, 1H), 3.70 (s, 3H), 3.75 (s, 3H).
[0053] 1.2 Split reaction
[0054] Tak...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com