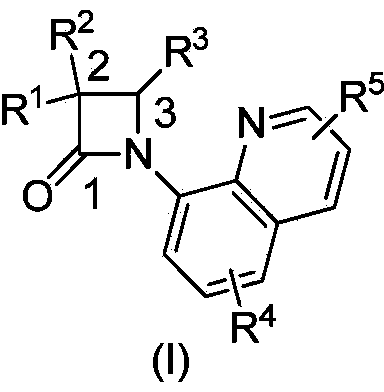
N-quinolyl substituted beta-lactam compound, as well as pharmaceutical composition, synthetic method and application of compound
A technology based on lactams and quinolines, applied in the field of pharmacy, can solve problems such as harsh conditions, use of toxic reagents, and low yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] (1) Preparation of 1-(8-quinolyl)-4-phenyl-azetidinone (1a):
[0082]
[0083] Operation is as follows: in the reaction tube of 10ml, add 1 (27.6mg, 0.1mmol) successively, Pd(OAc) 2 (1.1mg, 0.005mmol), AgOAc (20mg, 0.12mmol) and C 6 f 5 I (163mg, 0.55mmol), stirred evenly at room temperature, placed in a microwave reactor and heated to 160°C for 1.5 hours, purified by direct column chromatography (petroleum ether: ethyl acetate = 15:1) to obtain 25.5mg of compound 1a, 93% yield. solid; 1 HNMR (400MHz, CDCl 3 )δ8.77(s,1H),8.31(d,J=6.3Hz,1H),8.04(d,J=8.1Hz,1H),7.61–7.47(m,2H),7.41(d,J=7.0 Hz,2H),7.38–7.11(m,4H),6.27(s,1H),3.73(dd,J=15.0and5.2Hz,1H),3.11(d,J=14.7Hz,1H);13 C NMR (100MHz, CDCl 3 )δ166.8, 148.7, 140.5, 135.9, 133.2, 128.9, 128.5, 127.7, 126.5, 126.2, 124.4, 122.0, 121.3, 58.7, 47.8; HRMS (EI) Calcd for C 18 h 14 N 2 O[M + ]:274.1106,Found274.1113;IR(KBr)V(cm -1 ):1744,1638,1503,1344,756.
Embodiment 2
[0085] (2) Preparation of 1-(8-quinolyl)-4-(4-tolyl)-azetidinone (2a):
[0086]
[0087] Operation is as follows: in the reaction tube of 10ml, add 2 (29.0mg, 0.1mmol) successively, Pd(OAc) 2 (1.1mg, 0.005mmol), AgOAc (20mg, 0.12mmol) and C 6 f 5 I (163mg, 0.55mmol), stirred evenly at room temperature, placed in a microwave reactor and heated to 160°C for 1.5 hours, purified by direct column chromatography (petroleum ether: ethyl acetate = 15:1) to obtain 23.0mg of compound 2a, 80% yield. solid; 1 H NMR (400MHz, CDCl 3 )δ8.77(dd,J=4.1and1.7Hz,1H),8.27(dd,J=6.9and1.9Hz,1H),8.00(dd,J=8.3and1.6Hz,1H),7.54–7.44(m ,2H),7.35–7.22(m,3H),7.02(d,J=8.0Hz,2H),6.23(dd,J=5.5and2.5Hz,1H),3.70(dd,J=15.1and5.6Hz, 1H),3.09(dd,J=15.1and2.6Hz,1H),2.21(s,3H); 13 C NMR (100MHz, CDCl 3 )δ166.9, 148.7, 140.7, 137.5, 137.3, 136.0, 133.2, 129.2, 128.9, 126.6, 126.2, 124.5, 122.3, 121.3, 58.5, 47.7, 21.1; HRMS (EI) Calcd for C 19 h 16 N 2 O[M + ]:288.1263,Found288.1281;IR(KBr)V(cm -1 ):...
Embodiment 3
[0089] (3) Preparation of 1-(8-quinolyl)-4-(2-methoxyphenyl)-azetidinone (3a):
[0090]
[0091] The operation is as follows: add 3 (30.6mg, 0.1mmol), Pd(OAc) 2 (1.6mg, 0.007mmol), AgOAc (20mg, 0.12mmol) and C 6 f 5 I (163mg, 0.55mmol), stirred evenly at room temperature, placed in a microwave reactor and heated to 160°C for 1.5 hours, purified by direct column chromatography (petroleum ether: ethyl acetate = 12:1) to obtain 23.0mg of compound 3a, 82% yield. solid; 1 H NMR (500MHz, CDCl 3 )δ8.73(d,J=2.7Hz,1H),8.29(dd,J=7.0and1.5Hz,1H),8.02(d,J=8.2Hz,1H),7.58–7.45(m,2H), 7.30–7.25(m,1H),7.24(d,J=7.5Hz,1H),7.15–7.07(m,1H),6.80(d,J=8.2Hz,1H),6.71(t,J=7.5Hz ,1H),6.42(dd,J=5.7and2.6Hz,1H),3.87(s,3H),3.67(dd,J=14.9and5.8Hz,1H),3.13(dd,J=14.9and2.6Hz, 1H); 13 C NMR (100MHz, CDCl 3 )δ167.4, 157.1, 148.6, 140.8, 135.9, 133.9, 128.9, 128.5, 128.3, 127.4, 126.6, 124.1, 121.7, 121.2, 120.3, 110.4, 55.5, 55.4, 46.1; HRMS (EI) Calcd for C 19 h 16 N 2 o 2 [M + ]:304.1212,Fou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com