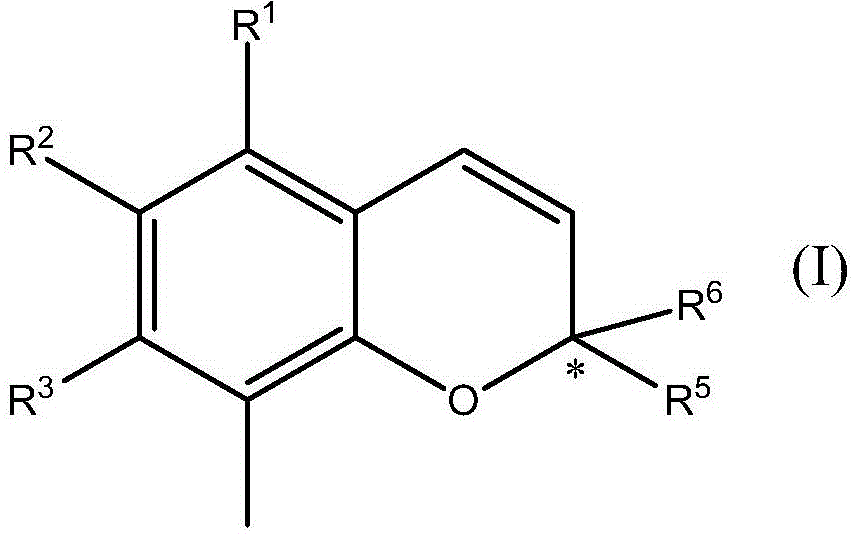
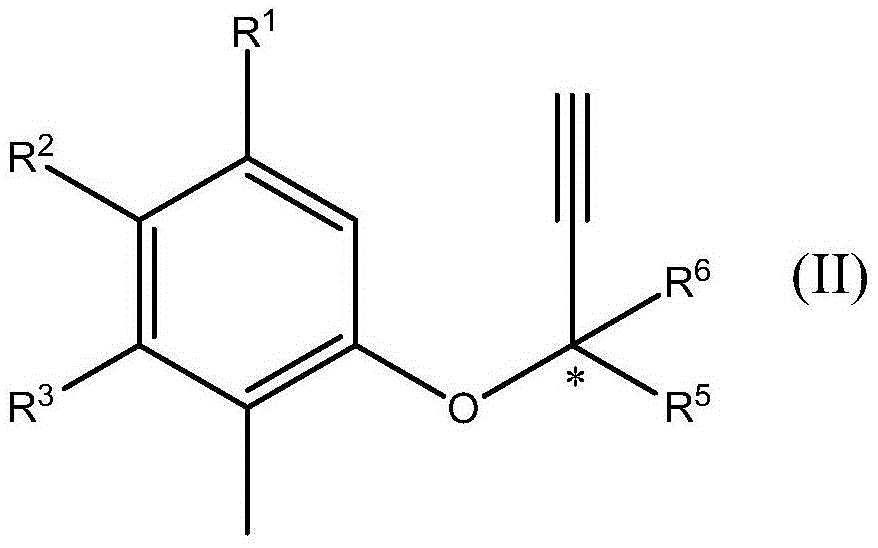
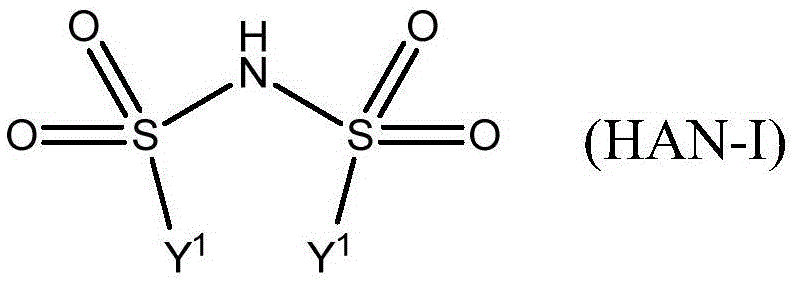
Formation of chromanes and chromenes by using silver(i) or gold(i) salts or complexes
A technology of complexes and compounds, applied in the direction of organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0198] The invention is further illustrated by the following experiments.
[0199] Analytical method
[0200] Gas Chromatography (GC):
[0201] GC analysis has been performed on an Agilent HP-6850 series system using FID-detector and using helium as a carrier gas. Separation was achieved on a HP-1 methylsiloxane (30 m x 0.32 mm, 0.25 μm) column using the following temperature programming: 50 °C (0 min) -> 10 °C / min -> 300 °C (5 min) . Samples were dissolved in ethyl acetate (5 mg sample in 1 mL ethyl acetate) and 1 μL of the solution was injected in a 50:1 split ratio.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com