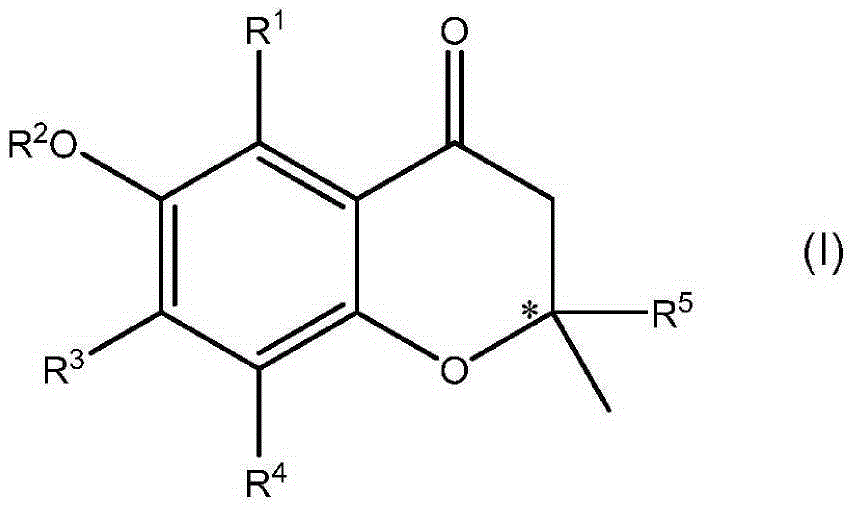
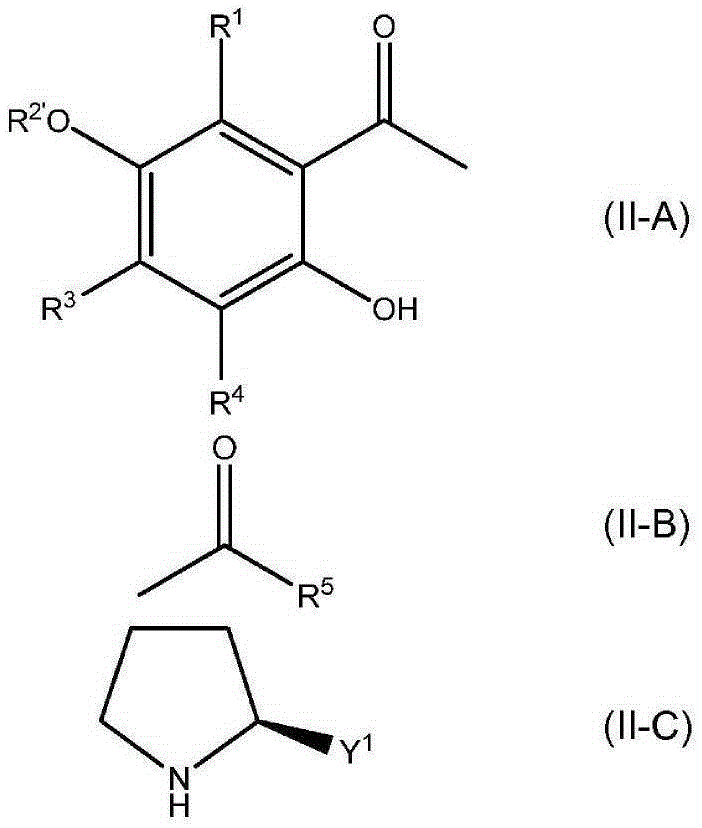
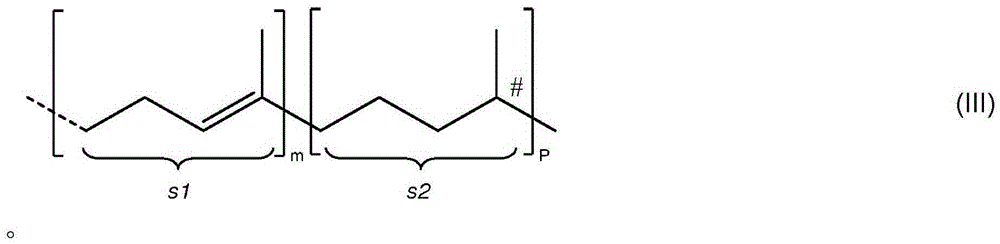
Formation of chiral 4-chromanone using chiral pyrrolidine in the presence of phenol or thiophenol
A technology of thiophenols and chiral centers, applied in organic chemistry, organic chemistry methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0168] The present invention is further illustrated by the following experiments.
[0169] use different additives
[0170] At 23 °C (or 40 °C), 0.5 mmol 2-acetyl- 3,5,6-trimethylhydroquinone (or 2-acetyl-3,5,6-trimethylhydroquinone 4-O-acetate) and 0.795 mmol of additives shown in Table 1 were suspended in 2.5 mL (23.47mmol) in toluene. Then, 0.514 mmol of E,E-farnesylacetone was added, and finally 0.795 mmol of (S)-2-(methoxymethyl)pyrrolidine was added. The reaction mixture was then stirred at 23°C (or 40°C) for the time indicated in Table 1 . When heated to 120°C, water was distilled off and the reaction mixture turned brown. After the indicated time at 120°C, the reaction mixture was cooled to 23°C. Then 1 mL of 2N aqueous HCl was added and the mixture was transferred to a separatory funnel and shaken well. The toluene phase was separated and washed with several 10 mL portions of water until a neutral aqueous phase was obtained. The organic layer was dried over so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com