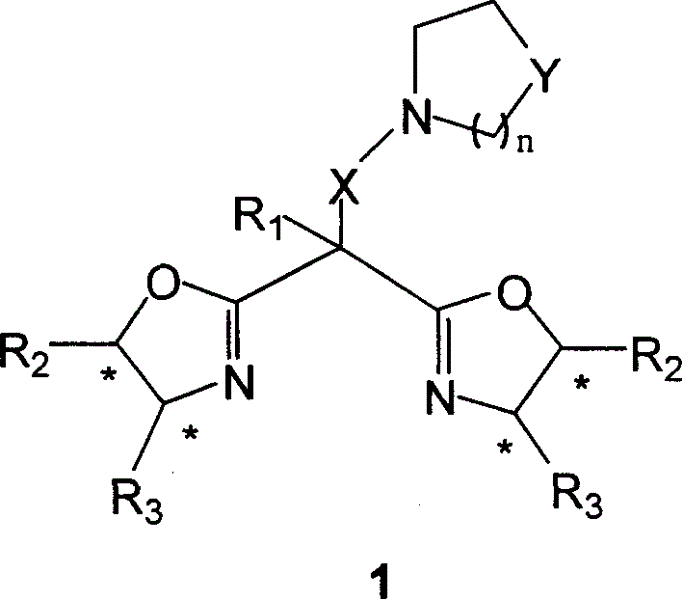
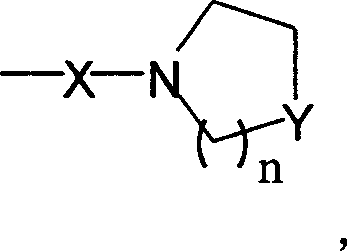Dual functions ligand compound of chirality dioxazoline, preparation and application
A ligand compound, bisoxazoline technology, applied in organic compound/hydride/coordination complex catalysts, chemical/physical processes, organic chemistry, etc., can solve bifunctional chiral bisoxazoline ligand reports There are few problems, such as few reports on the application of cyanosilylation reaction, etc., to achieve the effect of easy acquisition, easy preparation and good selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Synthesis of N-(3,3-dicyanopropyl)hexahydropyridine:
[0050] Add absolute ethanol (30 mL) and sodium (1.0 g, 43.5 mmol) into a 100 mL round bottom flask, and stir for 1 h. Then malononitrile (2.75 mL, 43.3 mmol) and potassium iodide (0.6 g, 3.6 mmol) were added and stirring was continued for 0.5 h. Then N-chloroethylhexahydropyridine hydrochloride (8.43 g, 45.8 mmol) was added and the reaction mixture was refluxed for 24 h. Water (10 mL) was added to the reactant, and extracted with chloroform (20 ml×2). The combined organic layers were washed with anhydrous Na 2 SO 4 After drying and concentrating, the crude product was obtained. Silica gel column chromatography (petroleum ether / chloroform 1:4) gave 3.82 g of N-(3,3-dicyanopropyl)hexahydropyridine, with a yield of 50%. IR: 2938, 2854, 2806, 2256, 1470, 1455, 1444, 1379, 1353, 1156, 1126, 1039cm -1 ; 1 H NMR (300MHz, CDCl 3 ): δ4.18(t, J=7.3Hz, 1H), 2.53(t, J=7.1Hz, 2H), 2.39(s, 4H), 2.13-2.18(m, 4H), 1.54-1.59(...
Embodiment 2
[0052] Synthesis of 1,1-bis[(4S)-4-phenyl-1,3-oxazolin-2-yl]-3-(1-piperidinyl)propane (1a):
[0053] Add anhydrous zinc chloride (50mg, 0.37mmol) into a 100mL two-neck flask, heat to melt, and cool to room temperature under nitrogen protection. Then chlorobenzene (20ml) and N-(3,3-dicyanopropyl)piperidine (0.55g, 3.1mmol) and L-phenylglycinol (0.93g, 6.82mmol) were added, and the mixture was refluxed for 24h. The solvent was distilled off under reduced pressure to obtain an oil, to which dichloromethane (20 mL) was added. The solution was extracted with water (20 mL×3), and the aqueous phase was extracted with dichloromethane (20 ml). The organic phases were combined and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure, and the oily residue was purified by silica gel column chromatography (eluent petroleum ether / chloroform 4:1) to obtain 1,1-bis[(4S)-4-phenyl-1,3-oxazoline -2-yl]-3-(1-piperidinyl)propane 0.59 g (46%). [α] D 20 = -...
Embodiment 3
[0055] Synthesis of 1,1-bis[(4S)-4-isobutyl-1,3-oxazolin-2-yl]-3-(1-piperidinyl)propane (1a):
[0056] Replace the L-phenylglycine alcohol in the embodiment two with L-leucinol, all the other are the same as the embodiment two, obtain 1,1-bis[(4S)-4-isobutyl-1,3-oxazoline- 2-yl]-3-(1-piperidinyl)propane, yield 48%.[α] D 20 = -30.3° (c0.7, CH 3 Oh)
[0057] IR: 2956, 2870, 1749, 1656, 1542, 1468, 1367, 1256, 1067cm -1 . 1 H NMR (CDCl 3 ): δ4.21-4.27(m, 2H), 4.02-4.07(m, 2H), 3.72-3.78(m, 2H), 3.36-3.40(t, J=7.1Hz, 1H), 2.20-2.29(m , 4H), 1.98-2.03(m, 2H), 1.61-1.65(m, 2H), 1.47-1.52(m, 6H), 1.32(m, 2H), 1.15-1.22(m, 2H), 0.81-0.85 (m, 12H). 13 C NMR (50MHz, CDCl 3 ): 22.53, 22.59, 22.78, 22.83, 24.34, 25.24, 25.88, 26.91, 37.69, 45.30, 45.39, 54.41, 56.50, 64.43, 64.47, 73.15, 73.25, 164.37, 164.42. MS (EI): 37 + , 2), 279(35), 249(12), 209(20), 168(18), 98(100).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com