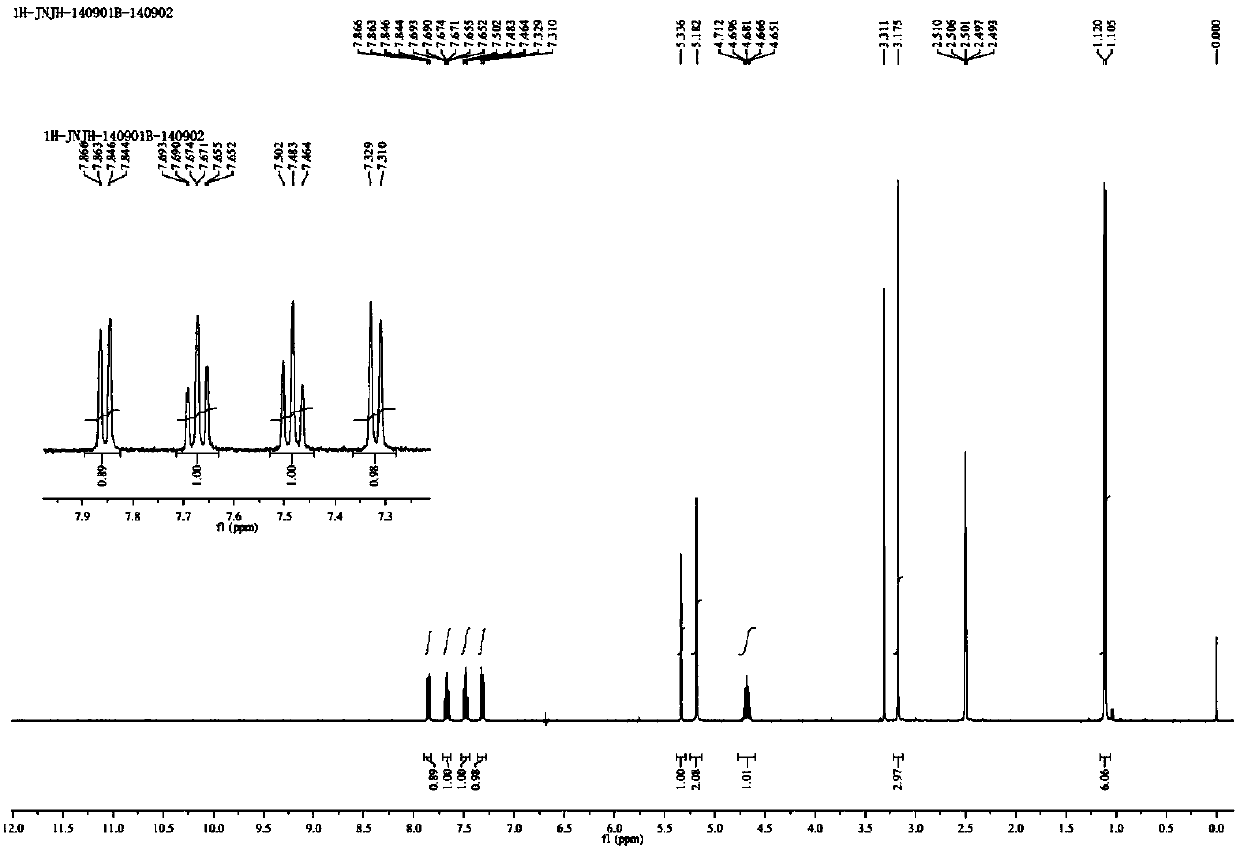
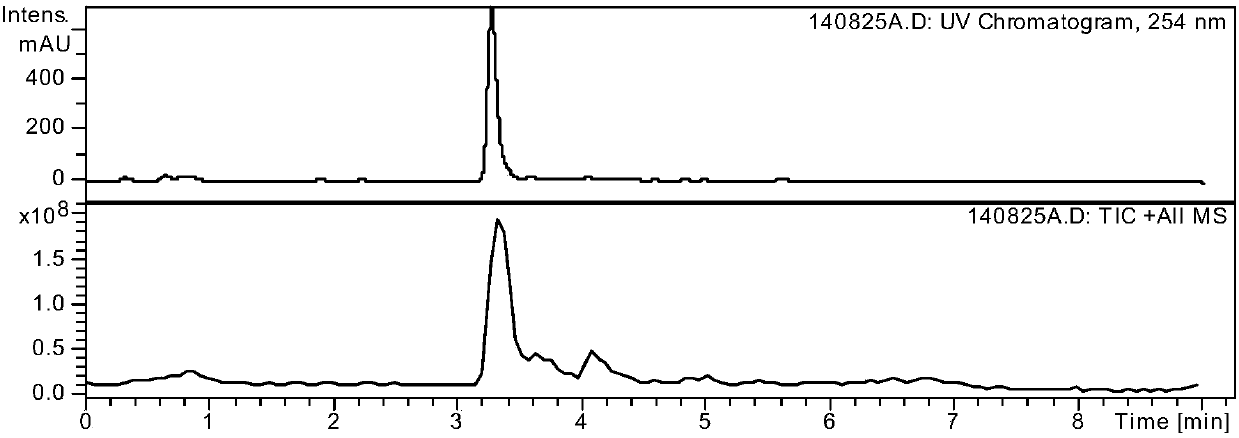
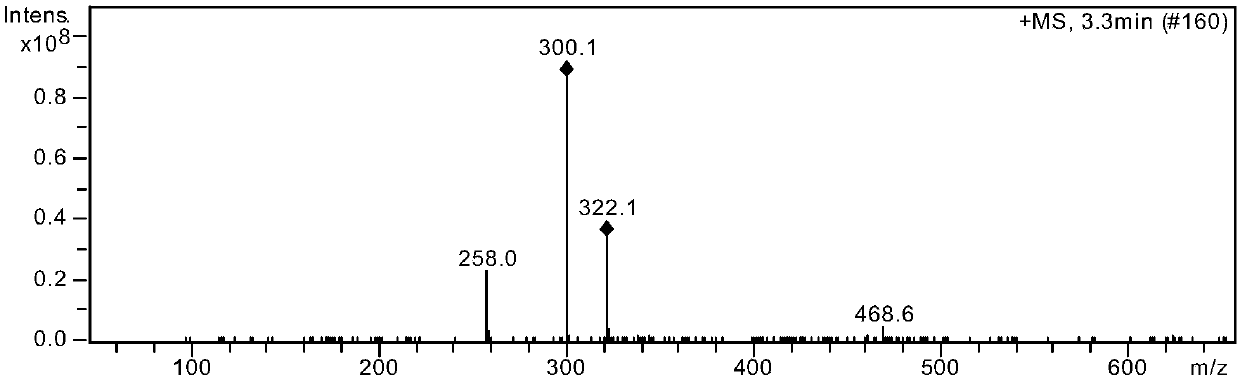
Preparation method of alogliptin benzoate impurity
A technology for benzoic acid and impurities is applied in the field of preparation of alogliptin benzoate impurities, and achieves the effects of mild and controllable reaction conditions, reduced side reactions and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] At 25°C, add 10.0g (62.3mmol) SM1, 40mL N-methylpyrrolidone, and 12.1g (93.6mmol) diisopropylethylamine into a 500mL three-necked flask in sequence, and then add the solution dropwise into the reaction flask under stirring. There is 40 mL of toluene solution of 13.4 g (68.4 mmol) SM2, after the dropwise addition, the temperature is raised to 70° C., and the reaction is carried out for 1.5 hours. The reaction system was lowered to room temperature, 40 mL of water was added thereto, stirred at 25° C. for 30 minutes, cooled to 0° C., stirred for 1 hour, and suction filtered. The filter cake was rinsed with 10 mL of isopropanol and then dried to obtain 15.5 g of off-white solid with a yield of 90.2%.
[0029] Add 5.5g (20mmol, 1eq) TM1 into 100mL tert-butanol, drop into 50mL aqueous solution of 5.0g (60mmol, 3eq) sodium bicarbonate at room temperature 25°C, and stir at room temperature 70°C for 12 hours.
[0030] Add concentrated hydrochloric acid dropwise to the system in...
Embodiment 2
[0032] The preparation of TM1 is the same as in Example 1.
[0033] Add 5.5g (20mmol, 1eq) TM1 into 100mL isopropanol, drop into 50mL aqueous solution of 5.0g (60mmol, 3eq) sodium bicarbonate at room temperature 25°C, and stir at room temperature 68°C for 16 hours.
[0034] Add concentrated hydrochloric acid dropwise to the system in a cold water bath at 10°C, adjust the pH to 5-6, remove isopropanol by rotary evaporation in a water bath at 40°C, add 100mL water and 100mL dichloromethane, separate the liquids, and extract the water phase with 100mL dichloromethane After three times, the combined organic phases were dried with anhydrous sodium sulfate, and the solvent was removed by rotary evaporation in a water bath at 40°C to obtain a brown-red oil. Add 20mL of dichloromethane to dissolve the oil, add 80mL of isopropyl ether, freeze at -10°C, a solid precipitates, filter and dry at 40°C to obtain 1.4g of a yellow solid, content 99.1%, yield 23%.
Embodiment 3
[0036] The preparation of TM1 is the same as in Example 1.
[0037] Add 5.5g (20mmol, 1eq) TM1 into 100mL ethanol, drop into 50mL aqueous solution of 5.0g (60mmol, 3eq) sodium bicarbonate at room temperature 25°C, and stir at room temperature 73°C for 8 hours.
[0038] Add concentrated hydrochloric acid dropwise to the system in a cold water bath at 10°C, adjust the pH to 5-6, remove ethanol by rotary evaporation in a water bath at 40°C, add 100mL water and 100mL dichloromethane, separate the layers, extract the water phase with 100mL dichloromethane three times, After the combined organic phases were dried with anhydrous sodium sulfate, the solvent was removed by rotary evaporation in a water bath at 40°C to obtain a brown-red oil. Add 20mL of dichloromethane to dissolve the oil, add 80mL of isopropyl ether, freeze at -10°C, a solid precipitates, filter and air-dry at 40°C to obtain 2.1g of a yellow solid, content 98.8%, yield 34.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com