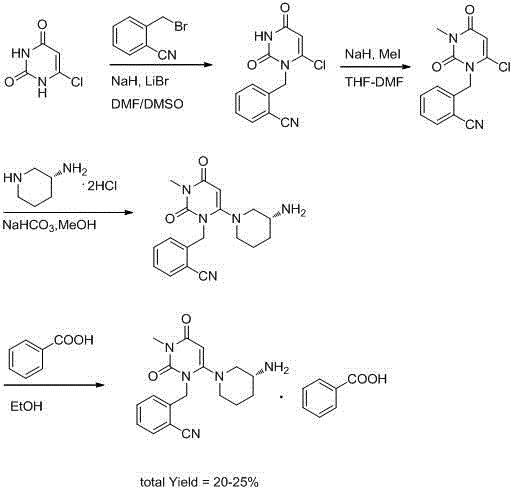
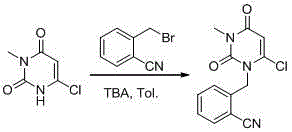
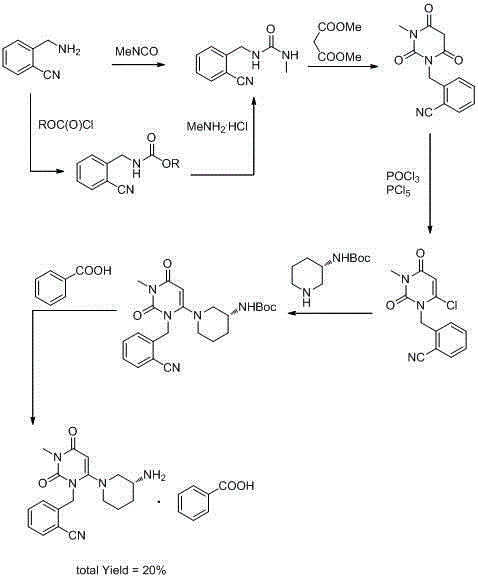
Preparation method for benzoic acid alogliptin polycrystalline type crystal
A technology of benzoic acid and polymorphs, which is applied in the field of preparation of alogliptin benzoate polymorph crystals, can solve the problems of insufficient purity and inconsistent crystal forms, and achieve the effects of high purity, easy control, and convenient industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A preparation method of alogliptin benzoate polymorphic crystal, the steps are:
[0042] (1) Add methanol to the reaction kettle and stir, then add the synthetic alogliptin benzoate crude product, heat and reflux for 15 minutes, press filter into the crystallization kettle of the fine drying bag and stir, and then press into the crystallization kettle Butyl ether, naturally cooled to room temperature, stirred and crystallized for 3 hours; the consumption of described methanol was equivalent to 4.5 times of the crude product quality of alogliptin benzoate; the consumption of described methyl tert-butyl ether was equivalent to benzoic acid 2.5 times the mass of alogliptin crude product;
[0043] (2) Centrifuge, wash the obtained solid with methanol, and dry at 45° C. to obtain alogliptin benzoate polymorphic crystal product with a yield of 69.1% and a purity of 99.99% by HPLC.
Embodiment 2
[0045] A preparation method of alogliptin benzoate polymorphic crystal, the steps are:
[0046] (1) Add methanol into the reaction kettle and stir, then add the synthetic alogliptin benzoate crude product, heat and reflux for 30 minutes, press filter into the crystallization kettle of the fine drying bag and stir, and then press into the crystallization kettle Butyl ether, naturally cooled to room temperature, stirred and crystallized for 4 hours; the consumption of described methanol was equivalent to 7 times of the quality of alogliptin benzoate crude product; the consumption of described methyl tert-butyl ether was equivalent to benzoic acid 7 times the mass of alogliptin crude product;
[0047] (2) Centrifuge, wash the obtained solid with methanol, and dry it with air blow at 55°C to obtain alogliptin benzoate polymorphic crystal product with a yield of 74.7% and a purity of 99.98% by HPLC.
[0048]
Embodiment 3
[0050] A preparation method of alogliptin benzoate polymorphic crystal, the steps are:
[0051] (1) Add methanol into the reaction kettle and stir, then add the synthetic alogliptin benzoate crude product, heat and reflux for 20 minutes, press filter into the crystallization kettle of the fine drying bag and stir, and then press into the crystallization kettle Butyl ether, naturally cooled to room temperature, stirred and crystallized for 3.5 hours; the consumption of described methanol was equivalent to 5 times of the crude product quality of alogliptin benzoate; the consumption of described methyl tert-butyl ether was equivalent to benzoic acid 4.5 times the mass of alogliptin crude product;
[0052] (2) Centrifuge, wash the obtained solid with methanol, and blow dry at 50°C to obtain alogliptin benzoate polymorphic crystal product with a yield of 81.6% and a purity of 99.99%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com