Benzoic acid alogliptin composition troche and preparation method thereof
A technology of benzoic acid and composition, applied in the field of alogliptin benzoate composition tablet and preparation thereof, can solve the problems of insufficient insulin secretion, slow disintegration speed, inconvenient use and the like, and achieves easy control of the technological process, Fast disintegration and easy portability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
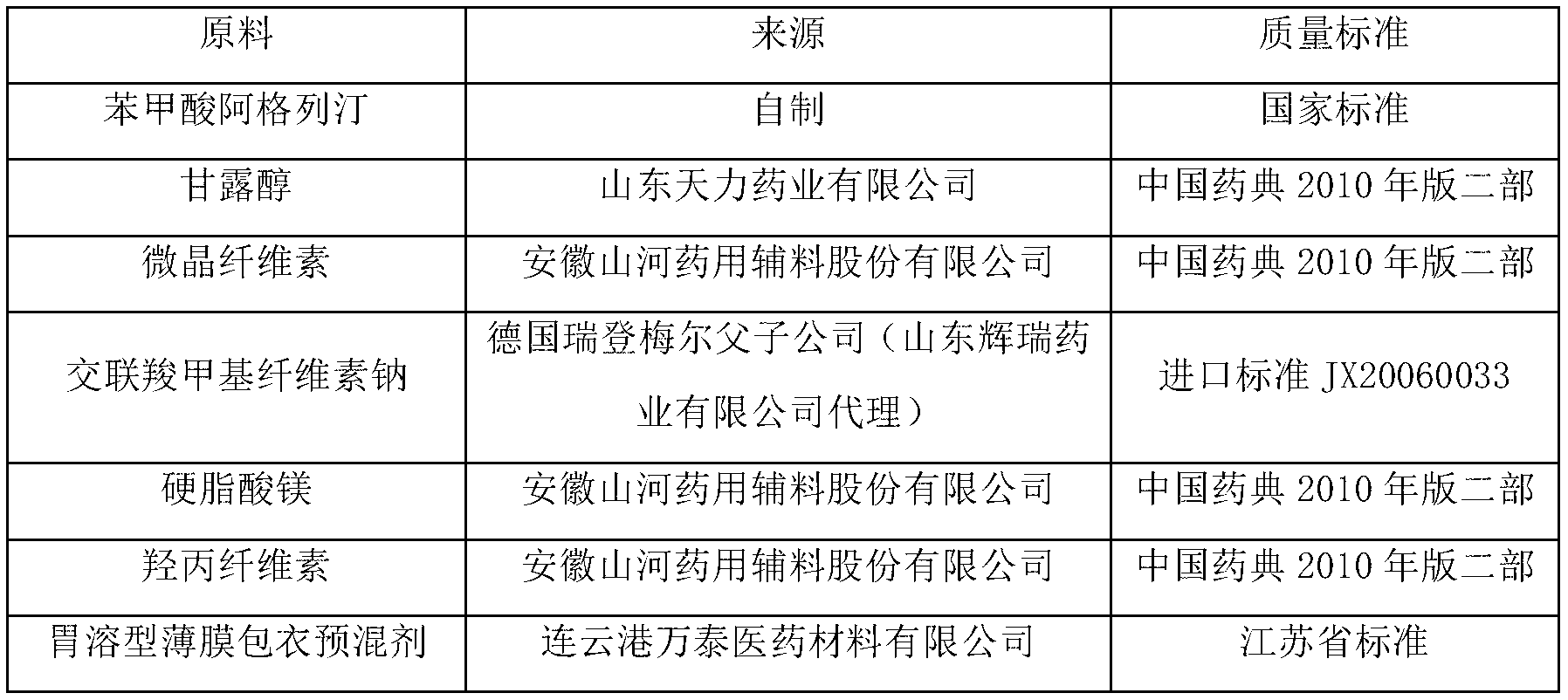
[0040] Produce 1000 specifications and be the alogliptin benzoate composition tablet of 25mg, the medicine active ingredient of described alogliptin benzoate composition tablet is alogliptin benzoate, mannitol, microcrystalline cellulose, The dosages of croscarmellose sodium, magnesium stearate and 5% hydroxypropyl cellulose aqueous solution are shown in Table 2.
[0041]Concrete preparation steps are as follows:
[0042] The first step: sieving, passing alogliptin benzoate through a 100-mesh sieve, and passing mannitol, microcrystalline cellulose and croscarmellose sodium through a 80-mesh sieve respectively;
[0043] The second step: mixing, mixing the sieved alogliptin benzoate, mannitol, microcrystalline cellulose and croscarmellose sodium evenly to obtain the first mixture, set aside;
[0044] The third step: granulation, adding the weighed 5% hydroxypropyl cellulose aqueous solution into the first mixture to make soft material, and granulating after passing through a 24...
Embodiment 2
[0053] Produce 1000 specifications and be the alogliptin benzoate composition tablet of 12.5mg, the drug active ingredient of described alogliptin benzoate composition tablet is alogliptin benzoate, mannitol, microcrystalline cellulose , croscarmellose sodium, magnesium stearate and 5% hydroxypropyl cellulose aqueous solution, the consumption of each raw material is as shown in table 4.
[0054] Concrete preparation steps are as follows:
[0055] The first step: sieving, passing the active ingredient alogliptin benzoate through a 100-mesh sieve, and passing mannitol, microcrystalline cellulose and croscarmellose sodium through a 80-mesh sieve;
[0056] The second step: mixing, mixing the sieved alogliptin benzoate, mannitol, microcrystalline cellulose and croscarmellose sodium evenly to obtain the first mixture, set aside;
[0057] The third step: granulation, adding the weighed 5% hydroxypropyl cellulose aqueous solution into the first mixture to make soft material, and gran...
Embodiment 3
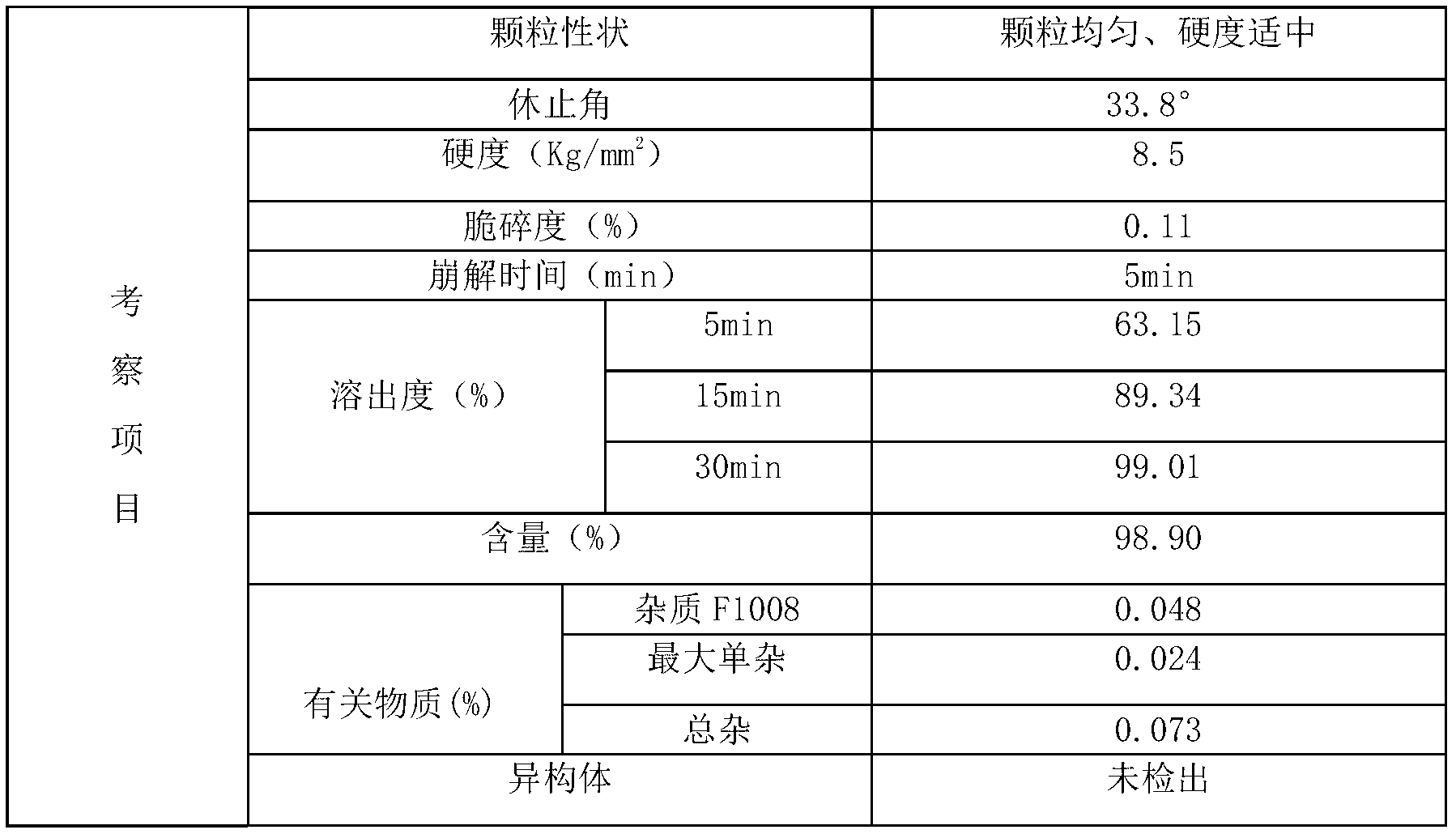
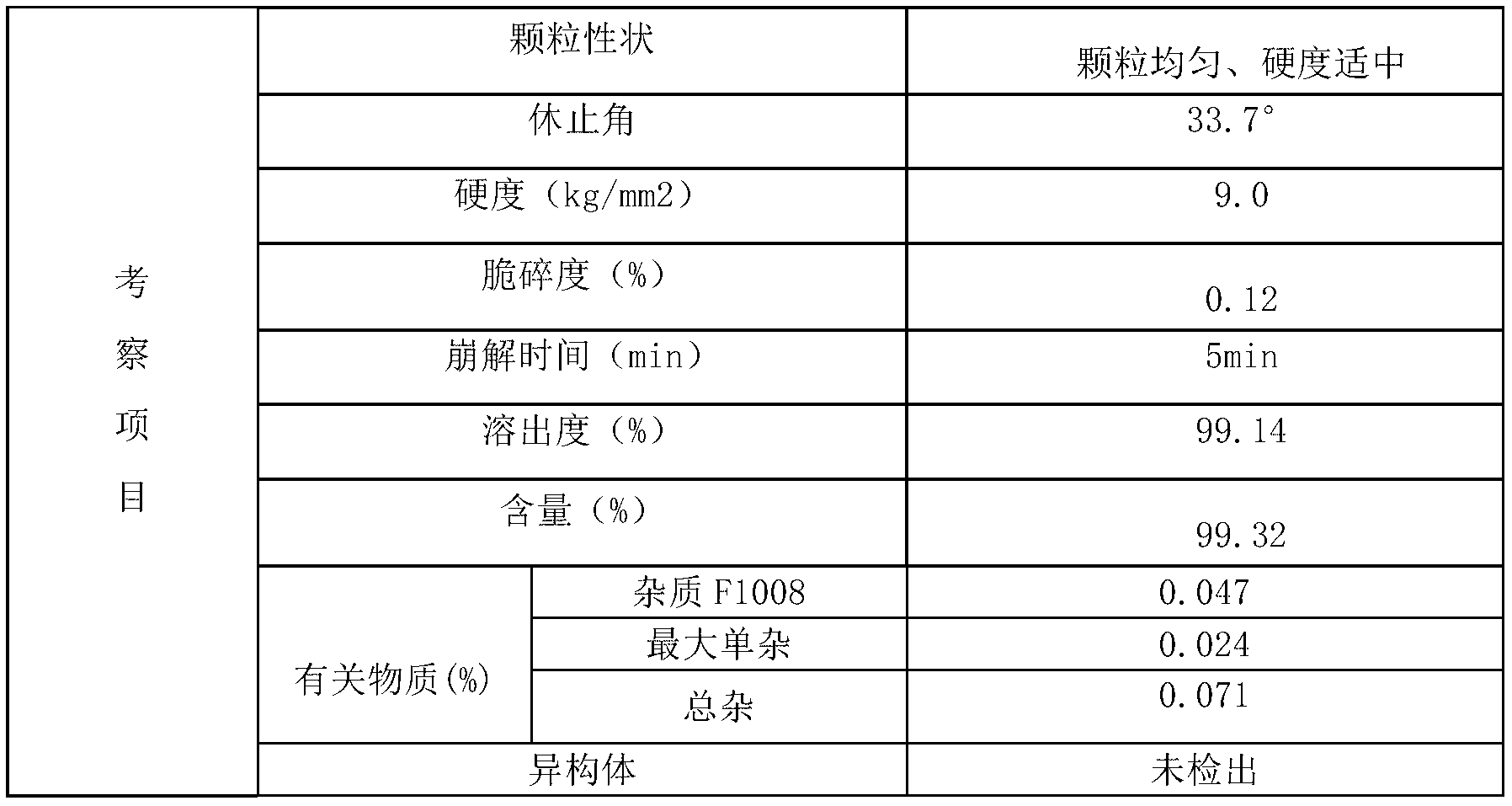
[0065] Adopt the alogliptin benzoate composition tablet of embodiment 1 to make the alogliptin benzoate composition film-coated tablet that specification is 25mg, formula is as follows:
[0066]
[0067] The preparation method is as follows:
[0068] Step 1: Weigh the Opadry coating powder and water, mix to make a coating solution, and set aside;
[0069] Step 2: Weigh the alogliptin benzoate composition tablet and put it in the coating pot, adjust the rotating speed of the coating pot to 5-10 rpm, control the inlet air temperature to 55-65°C and blow hot air to make the plain tablet temperature When it reaches 35-45°C, spray the coating liquid described in step (1) until the coating weight increases by 2.0% to 4.0%, stop spraying the coating liquid, and decide whether to continue to rotate the coating pan according to the degree of adhesion of one side, and then proceed to drying , to obtain the alogliptin benzoate composition film-coated tablet.
[0070] Influencing fac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com