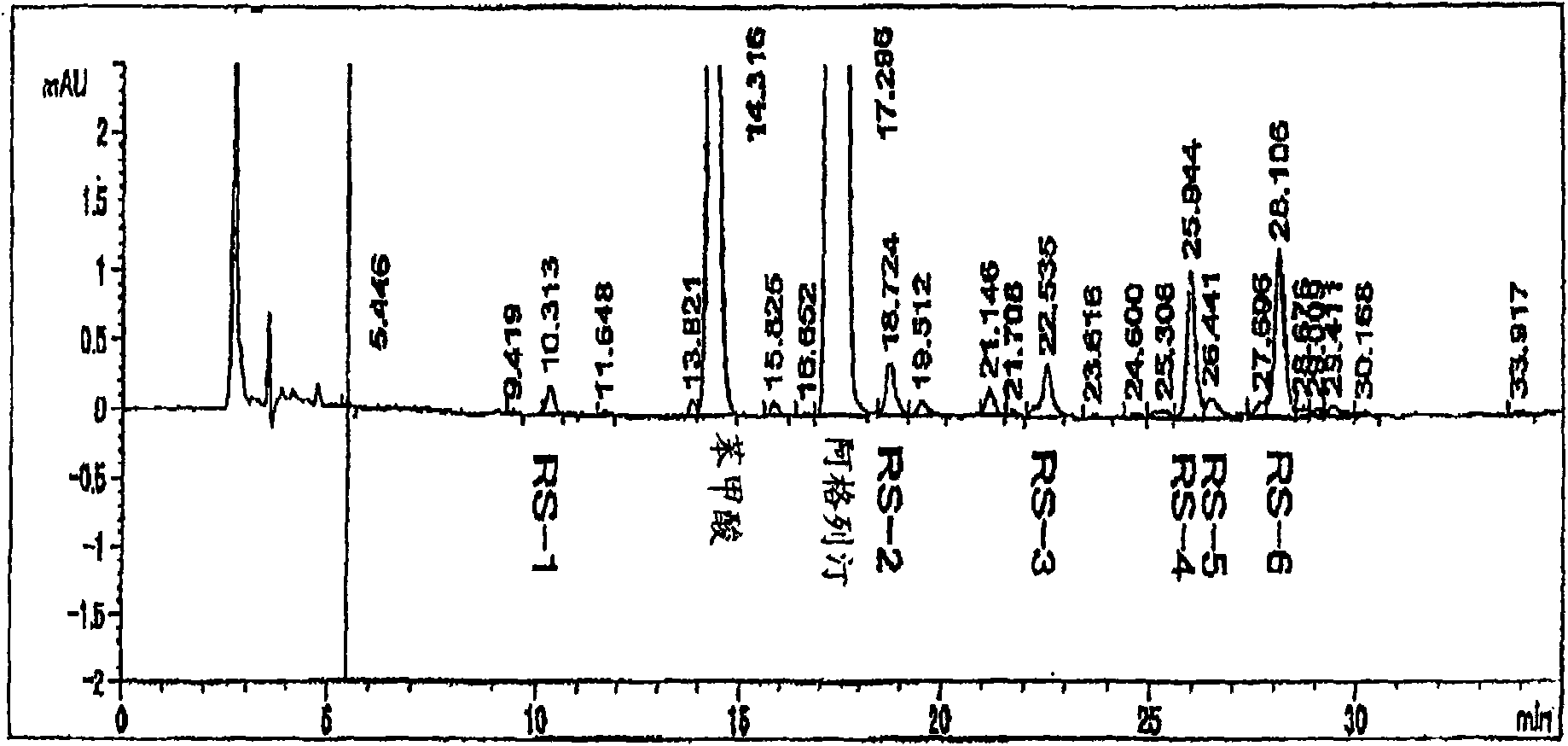
Solid preparation comprising alogliptin and metformin hydrochloride
A technology of metformin hydrochloride and solid preparations, which can be applied to medical preparations containing active ingredients, pill delivery, coatings, etc., and can solve problems such as storage stability of the compound metformin that has not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
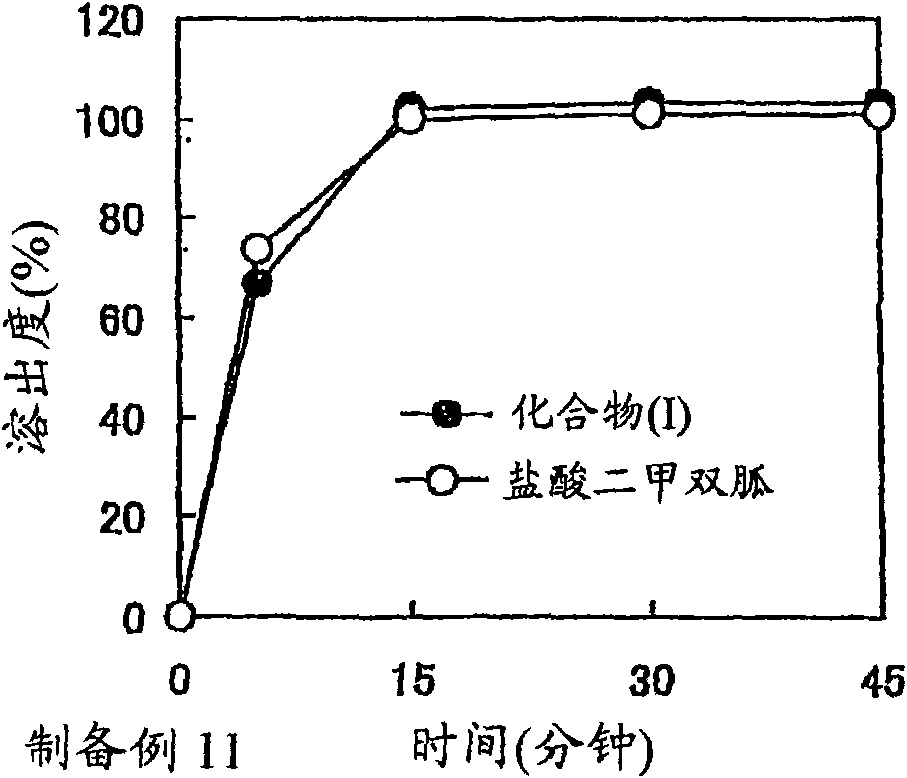
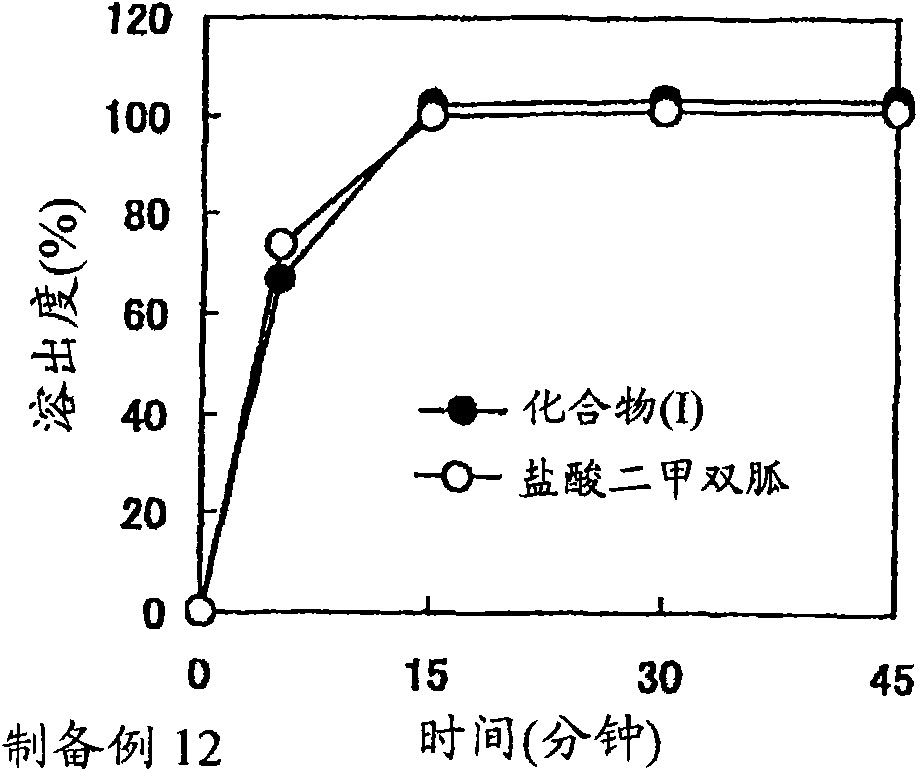
preparation example Construction
[0204] During the preparation of the coated tablet (A), it is preferable to form an inactive intermediate layer between the inner core and the outer layer to avoid direct contact between the inner core and the outer layer. The intermediate layer contains, for example, the above-mentioned coating base and coating additives. The intermediate layer preferably comprises an aqueous film coating base (preferably hydroxypropylmethylcellulose) and an opacifier and / or colorant (preferably talc).
[0205] In the above-mentioned coated tablet (A), the outer layer is formed at a ratio of preferably 0.1-100 parts by weight, more preferably 1-50 parts by weight, still more preferably 3-30 parts by weight, relative to 100 parts by weight of the inner core.
[0206] Moreover, in the above-mentioned coated tablet (A), the intermediate layer is formed at a ratio of preferably 0.1-30 parts by weight, more preferably 0.5-20 parts by weight, still more preferably 1-5 parts by weight, relative to 1...
preparation example 1
[0278] A mixture of benzoate (177.08 g), mannitol (262.5 g) and crystalline cellulose (31.25 g) of compound (I) was subjected to fluidized bed granulation (POWREX CORPORATION, model: LAB-1), while spraying An aqueous solution (292 g) of polyvinylpyrrolidone (29.18 g) was obtained to obtain granules containing compound (IA).
[0279] Separately, a mixture of metformin hydrochloride (463.0 g) and crystalline cellulose (11.11 g) was subjected to fluidized bed granulation (POWREX CORPORATION, model: LAB-1), while spraying an aqueous solution (259 g) of polyvinylpyrrolidone (25.93 g) To obtain granules containing metformin hydrochloride.
[0280] The above compound (IA)-containing granules (9.6 g), metformin hydrochloride-containing granules (432.2 g), crystalline cellulose (CEOLUS KG-802, 25.1 g), crospovidone (Kollidon CL-F, 24.63 g) Mixed with magnesium stearate (1.48 g) to obtain a powder mixture. The resulting powder mixture was tableted using a rotary tablet press (KIKUSUI ...
preparation example 2
[0283] A mixture of benzoate (347.01 g), mannitol (1428.6 g) and crystalline cellulose (122.48 g) of compound (I) was subjected to fluidized bed granulation (POWREX CORPORATION, model: FD-3S), while spraying Aqueous solution (1020 g) of polyvinylpyrrolidone (102.06 g) to obtain granules containing compound (IA).
[0284] Separately, a mixture of metformin hydrochloride (1851.9 g) and crystalline cellulose (44.44 g) was subjected to fluidized bed granulation (POWREX CORPORATION, model: FD-3S), while spraying an aqueous solution (1037 g) of polyvinylpyrrolidone (103.7 g) To obtain granules containing metformin hydrochloride.
[0285] The above compound (IA)-containing granules (58.8 g), metformin hydrochloride-containing granules (648 g), crystalline cellulose (CEOLUS KG-802, 39.6 g), crospovidone (Kollidon CL-F, 39.6 g) and Magnesium stearate (2.4 g) was mixed to obtain a powder mixture. The resulting powder mixture was tableted using a rotary tablet press (KIKUSUI SEISAKUSYO...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com