Preparation method of indoline derivative for synthesizing silodosin
A technology of silodosin and indoline, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of poor reductive amination selectivity, unfavorable scale-up production, and high production costs, and achieves improved chiral purity, avoiding loss, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
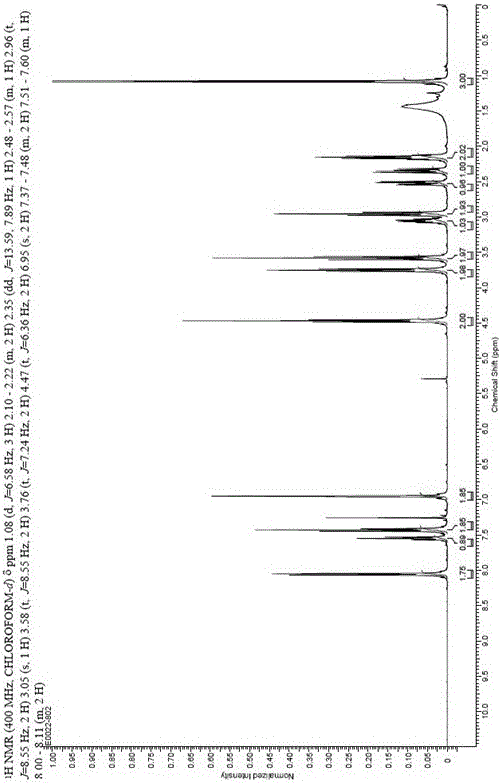
[0037] Example 1: Compound (1): Preparation of 1-(3-benzoyloxypropyl)indoline;
[0038] Add 26.8g of benzoic acid, 90ml of DMF, 30.6ml of triethylamine and 21.7ml of 1-chloro-3-bromopropane into the reaction flask, stir at 25°C for 12 hours, raise the temperature to 50°C and stir for 3 hours, add 23.6ml of indoline, 30.6 ml of triethylamine, react at 100°C for 6 hours. Cool to room temperature, add 180ml of water, extract twice with ethyl acetate, combine the ethyl acetate layers, wash with saturated sodium bicarbonate and saturated brine in turn, add 3mol / L dilute hydrochloric acid to extract to the water layer, add saturated sodium carbonate solution Adjust the pH=8-9, add dichloromethane to extract twice, wash with saturated saline, dry over anhydrous sodium sulfate, and recover the dry solvent under reduced pressure to obtain 46.4g of 1-(3-phenoxypropyl)indoline; yield : 78%, purity: 98.66%.
Embodiment 2
[0039] Example 2: Compound (2): Preparation of [1-(3-benzoyloxypropyl)-5-formyl]indoline.
[0040] Slowly add 30.8g of phosphorus oxychloride dropwise to 62.5ml of DMF under ice cooling, continue to stir for 30 minutes under the ice bath, and add 28.1g of 1-(3-benzyl) prepared in Example 1 in batches. Acyloxypropyl) indoline, heated to 25 ° C for 3 hours, slowly poured into 800ml of ice water, crystallized overnight, filtered the next day, and dried to obtain 31g of light yellow solid, namely [1-(3-benzene Formyloxypropyl)-5-formyl]indoline; yield: 92%, purity: 92.43%.
Embodiment 3
[0041] Example 3: Compound (3): Preparation of (1R,2R)-[1-(3-benzoyloxypropyl)-5-(1-hydroxyl-2-nitropropyl)]indoline .
[0042] Under nitrogen protection, add 0.91g copper acetate, 1.9g quinidine, 140ml ethanol to the three-necked reaction flask, stir at 25°C for 4 h, cool the resulting catalyst solution to about -10°C, add 31g of the compound prepared in Example 2 ( 2) Ethanol solution (120ml), keep below -10°C and add 37.5g of nitroethane dropwise after adding, keep warm at -10°C for 12 hours after dropping. Add 150ml of 1mol / L hydrochloric acid to quench the reaction after the reaction, concentrate under reduced pressure at 35~40°C to remove most of the ethanol, add ethyl acetate to extract three times, combine the organic phases, wash once with saturated saline, dry over anhydrous sodium sulfate, and filter Concentration gave 32.6g of oily substance, namely (1R,2R)-[1-(3-benzoyloxypropyl)-5-(1-hydroxy-2-nitropropyl)]indoline; Yield: 92%, purity: 98.73%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com