Preparation method of R-3-aminobutyric acid
A kind of aminobutyric acid, R-3- technology, applied in the field of biocatalysis, to achieve the effect of high product purity and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. Construction of Aspartase Cloning Strain
[0033] After the frozen bacillus was revived and cultured, a pair of primers were designed according to the sequence of the bacillus aspartase gene published on NCBI (such as GenBank: AB028242.1) using the bacterial solution as a DNA template:
[0034] Forward primer (ASP-P-BamH I): 5'-CGCGGATCCATGAATACCGATGTTCGT-3';
[0035] Reverse primer (ASP-R-Xho I): 5'-CCGCTCGAGTATTTTTCTTCCAGCAATTCCCGG-3';
[0036] PCR amplification was then carried out, and the reaction conditions were pre-denaturation at 94°C for 5 minutes, followed by 30 cycles (94°C for 30s, 59°C for 30s, 72°C for 1.5min), and 72°C for 10 minutes.
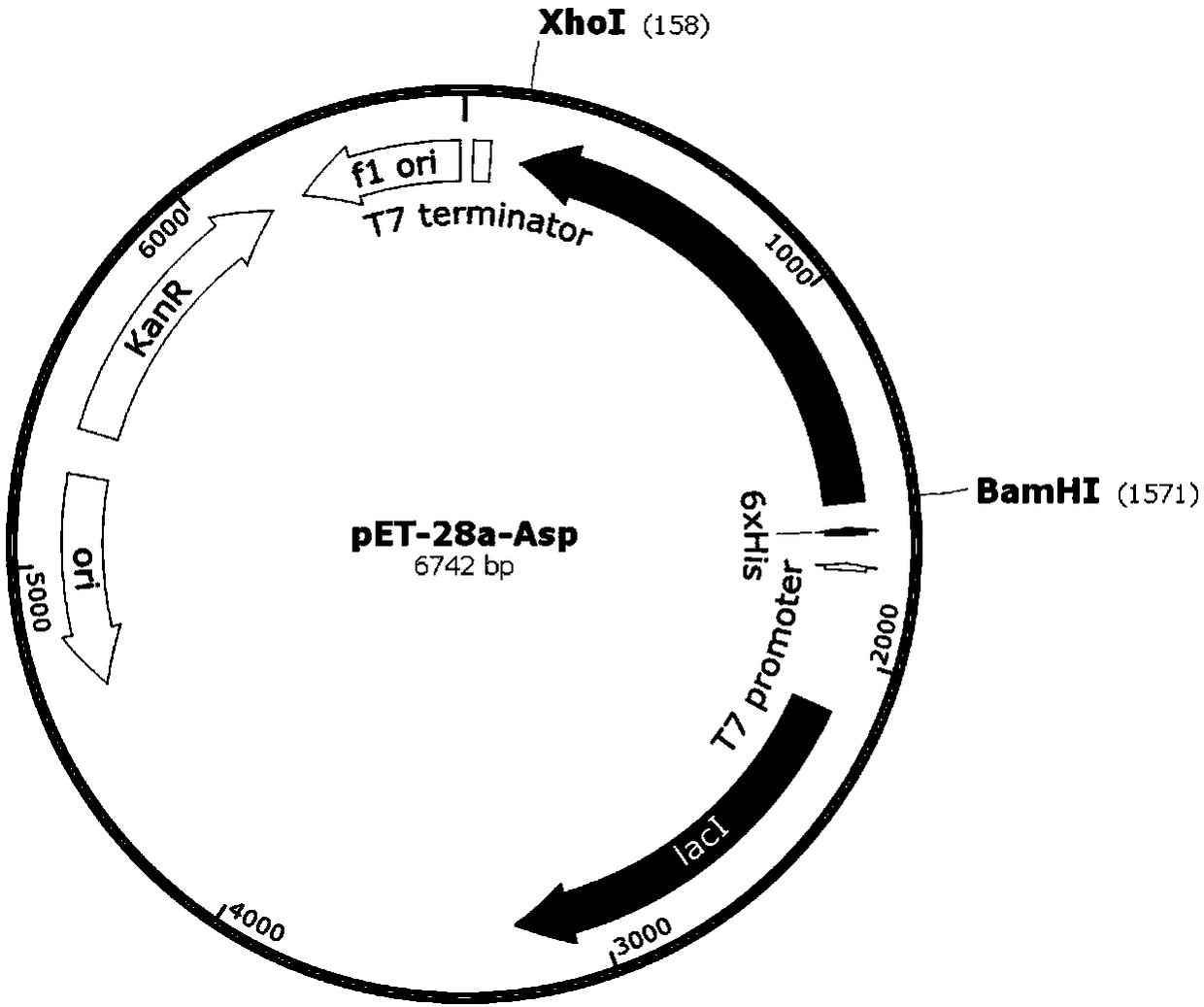
[0037]After the completion of PCR, gel verification and product recovery were carried out. Subsequently, the target fragment and the pET-28a(+) plasmid were digested by a double enzyme digestion system composed of BamH I and Xho I. After the reaction, the digested product was recovered, and then the digested target ...
Embodiment 2
[0044] Example 2 1000L system biocatalytic reaction
[0045] In the 1000L catalytic system, add 250kg of crotonic acid, 2.4kg of magnesium sulfate, 58kg of ammonium sulfate, then adjust the pH to 9.0 with ammonia water, stir at 40°C, add 10kg of aspartase sludge obtained in Example 1 above, and start reaction. After 12 hours of reaction, the concentration of R-3-aminobutyric acid in the solution can reach 294 g / L, and the conversion rate can reach more than 98%.
[0046] Please refer further Figure 3 to Figure 5 as shown, image 3 For adding the substrate and adjusting the pH, the liquid phase chromatogram, Figure 4 The liquid phase chromatogram of reaction 4h shows that the reaction speed is very fast. Figure 5 It is the liquid phase spectrum when reacting 12h, can find out from this figure that the substrate has been reacted substantially, and the residue of substrate is very low.
[0047] After the reaction is over, the reaction solution is treated with a microfiltr...
Embodiment 3
[0049] Example 3 1000L system biocatalytic reaction
[0050] In a 1000L catalytic system, add 270kg of crotonic acid, 2.4kg of magnesium sulfate, 72.5kg of ammonium acetate, adjust the pH to 9.2 with ammonia water, stir at 38°C, add 11kg of aspartase sludge, and start the reaction. After 12 hours of reaction, the concentration of R-3-aminobutyric acid in the solution can reach 319 g / L, and the conversion rate can reach more than 98%.
[0051] After the reaction is over, the reaction solution is treated with a microfiltration membrane to retain aspartase sludge and most of the enzyme protein, and the supernatant is passed through a nanofiltration membrane to remove SO 4 2- and most pigments. For microfiltration, microfiltration membranes with a relative molecular weight greater than 3000-5000 Daltons are selected for separation, and nanofiltration is used for separation with sodium filtration membranes with a relative molecular weight greater than 120-200 Daltons. The obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com