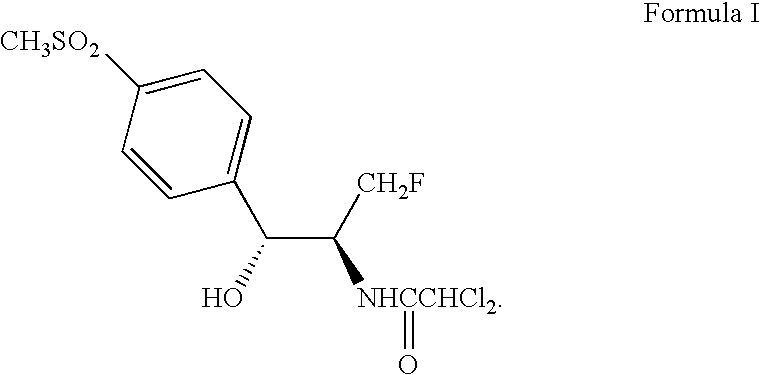
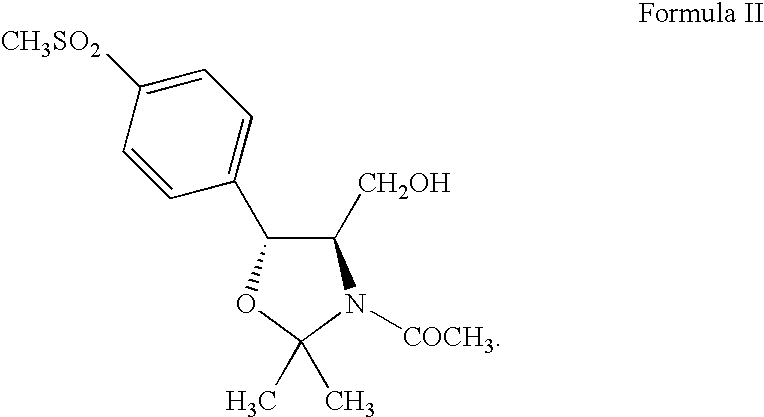
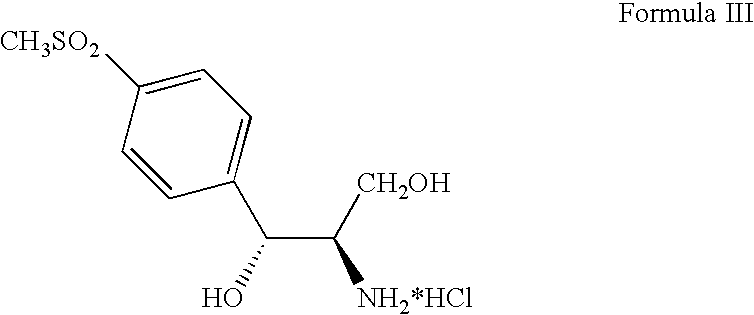
Process for preparing oxazolidine protected aminodiol compounds useful as intermediates to florfenicol
a technology of oxazolidine and aminodiol, which is applied in the preparation of organic compounds, chemical instruments and processes, and organic chemistry, etc., can solve the problems of difficult isolation and handling, high cost of aminodiol compound formula iii, and high cost of aminodiol compound, so as to improve yield, reduce cost, and process efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of (4R,5R)-3-acetyl-2,2-dimethyl-4-hydroxymethyl-5-[4-(methylsulfonyl)phenyl]-1,3-oxazolidine (Compound II)
[0093] (2S,3R)-Ethyl-2-amino-3-[4-(methylsulfonyl)phenyl]-3-hydroxy-propanoate (Compound IV) (100 g, 0.3480 moles) in 500 mL of methanol reacts with potassium borohydride (28.2 g, 0.5220 moles) over 4-8 hours at 50-60° C. to quantitatively yield (1R,2R)-2-amino-1-[4-(methylsulfonyl)phenyl]-1,3-propandiol (Compound VII: R1 is methylsulfonyl) (85.36 g, 0.3480 moles) in solution. Toluene (500 mL) and acetone (500 mL) replace methanol which distills off. Addition of potassium carbonate (6.9 g, 0.0696 moles) with heating at 75-85° C. for 12-18 hours yields (4R,5R)-2,2-dimethyl-4-hydroxymethyl-5-[4-(methylsulfonyl)phenyl]-1,3-oxazolidine (Compound VIII: R1 is methylsulfonyl and R2 and R3 are methyl). Addition of potassium carbonate (19.0 g, 0.1914 moles) and acetyl chloride (30.0 g, 0.3828 moles) at 20-25° C. for 2-4 hours then addition of water (500 mL) precipitates the...
example 2
Preparation of (4R,5R)-3-acetyl-2,2-dimethyl-4-hydroxymethyl-5-[4-(methylsulfonyl)phenyl]-1,3-oxazolidine (Compound II)
[0094] (2S,3R)-Ethyl-2-amino-3-[4-(methylsulfonyl)phenyl]-3-hydroxy-propanoate (Compound IV) (100 g, 0.3480 moles) in methanol (450 mL) reacts with potassium borohydride (28.2 g, 0.5220 moles) over 4-8 hours at 50-60° C. to quantitatively yield (1R,2R)-2-amino-1-[4-(methylsulfonyl)phenyl]-1,3-propandiol (Compound VII: R1 is methylsulfonyl) (85.4 g, 0.3480 moles) in solution. Toluene (450 mL) and acetone (450 mL) replace methanol which distills off. Addition of triethylamine (8.8 g, 0.0870 moles) with heating at 70-80° C. for 12-18 hours yields (4R,5R)-2,2-dimethyl-4-hydroxymethyl-5-[4-(methylsulfonyl)phenyl]-1,3-oxazolidine (Compound VII: R1 is methylsulfonyl and R2 and R3 are methyl). Addition of triethylamine (44.5 g, 0.4402 moles) and acetyl chloride (30.0 g, 0.3828 moles) at 20-25° C. for 2-4 hours then addition of water (500 mL) precipitates the crude product....
example 3
Preparation of (4R,5R)-3-acetyl-2,2-dimethyl-4-hydroxymethyl-5-[4-(methylsulfonyl)phenyl]-1,3-oxazolidine (Compound II)
[0095] (2S,3R)-Ethyl-2-amino-3-[4-(methylsulfonyl)phenyl]-3-hydroxy-propanoate (Compound IV) (100 g, 0.3480 moles) in tetrahydrofuran (500 mL) reacts with lithium aluminum hydride (16.0 g, 0.4224 moles) over 4-8 hours at 60-70° C. to quantitatively yield (1R,2R)-2-amino-1-[4-(methylsulfonyl)phenyl]-1,3-propandiol (Compound VII: R1 is methylsulfonyl) (85.36 g, 0.3480 moles). Addition of ethyl acetate (75 mL) destroys any excess lithium aluminum hydride. Addition of xylene (600 mL), 2-methoxypropene (37.6 g, 0.5220 moles), and p-toluenesulfonic acid monohydrate (6.6 g, 0.0348 moles) with agitation at 20-30° C. for 10-16 hours produces (4R,5R)-2,2-dimethyl-4-hydroxymethyl-5-[4-(methylsulfonyl)phenyl]-1,3-oxazolidine (Compound VIII: R1 is methylsulfonyl and R2 and R3 are methyl). Addition of triethylamine (81.3 g, 0.8039 moles) and acetyl chloride (30.0 g, 0.3828 moles...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com