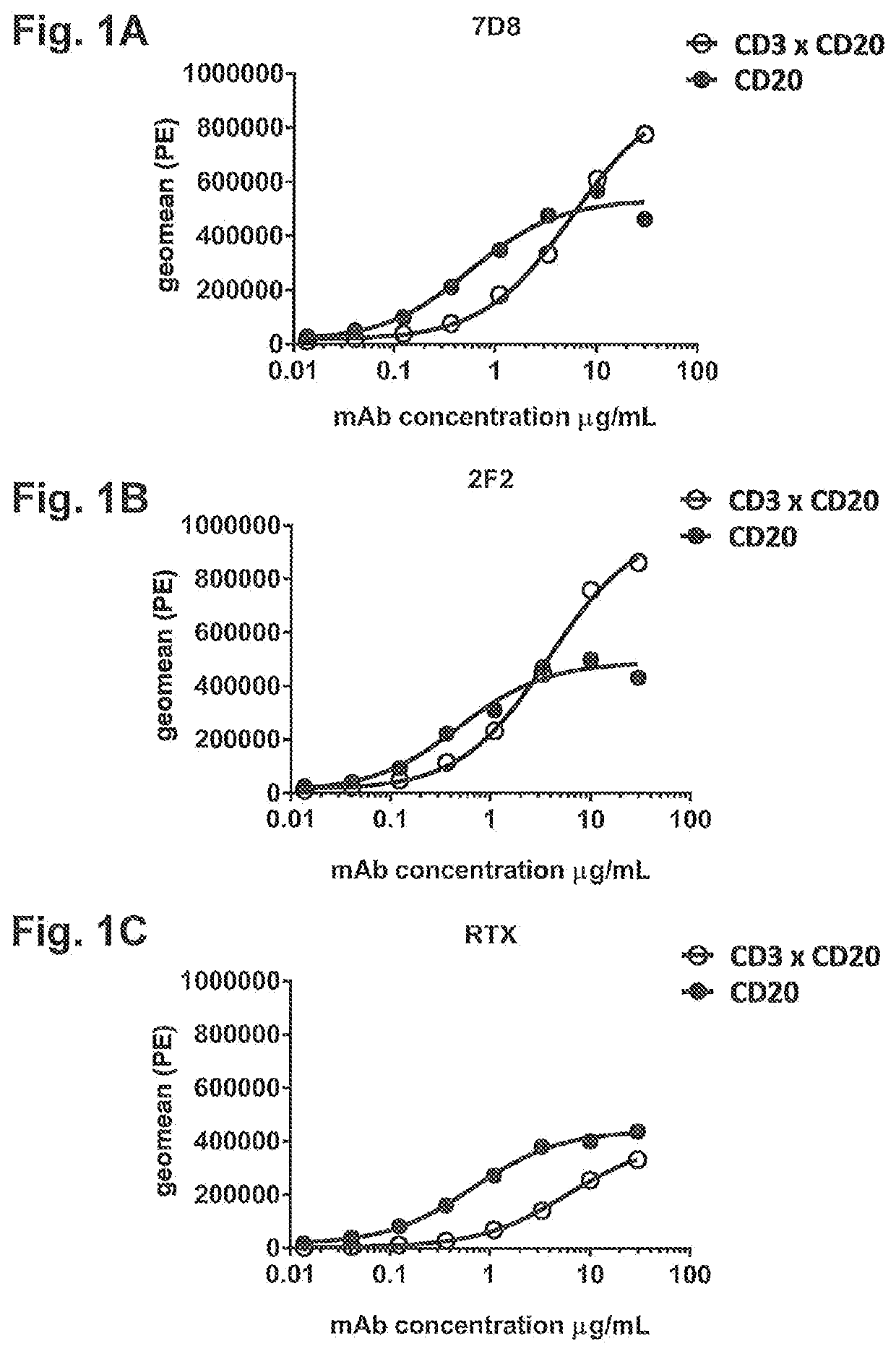
Bispecific antibodies against cd3 and cd20
a technology applied in the field of cd3 and cd20, can solve the problems of low efficacy and disappointing initial clinical studies, and achieve the effects of rapid and strong cell killing, high killing efficiency, and high potency in eradicating tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
n of Humanized CD3 Antibodies and Non-Activating Antibody Variants
Humanization of CD3 Antibodies
[0658]Humanization of a murine CD3 antibody SP34 (U.S. Pat. No. 8,236,308, described herein as IgG1-CD3) was performed by Antitope (Cambridge, UK) using their improved version of the germline humanization (CDR-grafting) technology (EP0629240). Using this technology, 4 different VH chains (SEQ ID NOs:6, 7, 8, and 9) and 3 different VL chains (SEQ ID NOs:10, 11, and 12) were designed. By combining these 4 VH with the 3 VL chains, 12 different antibodies were generated. The humanized variants are described herein as huCD3. Thus, humanized variants comprising a VH and a VL according to the invention, are described as, e.g., IgG1-huCD3-H1L1 meaning that said specific variant is of the IgG1 isotype, is a humanized SP34 CD3-specific antibody and comprises the VH amino acid sequence termed “H1” and is defined according to SEQ ID NO:6, and the VL amino acid sequence termed “L1” and is defined acco...
example 2
n of Bispecific Antibodies by 2-MEA-Induced Fab-Arm Exchange
[0681]An in vitro method for producing bispecific antibodies is described in WO2008119353 (Genmab) and reported by van der Neut-Kolfschoten et al. (Science. 2007 Sep. 14; 317(5844):1554-7). Herein, the bispecific antibodies were formed by “Fab-arm” or “half-molecule” exchange (swapping of a heavy chain and attached light chain) between two monospecific IgG4- or IgG4-like antibodies upon incubation under mildly reducing conditions. Without being limited to theory, this Fab-arm exchange reaction was the result of a disulfide-bond isomerization reaction wherein the inter heavy-chain disulfide bonds in the hinge regions of monospecific antibodies were reduced and the resulting free cysteines form a new inter heavy-chain disulfide bond with cysteine residues of another antibody molecule with a different specificity. The resulting products were bispecific antibodies having two Fab arms with different sequences.
[0682]The knowledge...
example 3
n of Mutants to Optimize the Production of the Humanized CD3 Antibodies
[0685]Generation of huCD3-L1 Mutant Plasmids
[0686]Several IgG1-huCD3-H1L1 variants with mutations in the L1 light chain were generated in order to improve the expression levels of IgG1-huCD3-H1L1 in transient transfection assays, cf. Table 5. The selection of residues was based on comparisons with germline sequences or screening for the presence of rare residues in the huCD3-L1 sequence in combination with crystal structures from homologous antibodies. The selected sequences were synthesized at GeneArt (Life Technologies, Germany). p33L encodes the constant domain of the human IgLC2 / IgLC3 lambda light chain of SEQ ID NO:29. p33Glf encodes the IgG1m(f) heavy chain constant region of SEQ ID NO: 15.
TABLE 5Antibody name after co-expressionLC constructsLC Mutantswith HC VH1 encoding plasmidp33L-huCD3-VL1—IgG1-huCD3-H1L1p33L-huCD3-VL1-F10LF10LIgG1-huCD3-H1L1-LF10Lp33L-huCD3-VL1-R23AR23AIgG1-huCD3-H1L1-LR23Ap33L-huCD3-V...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com