Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
81 results about "Acute lymphoblastic leukemias" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
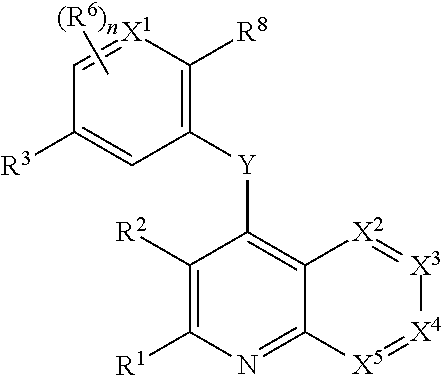
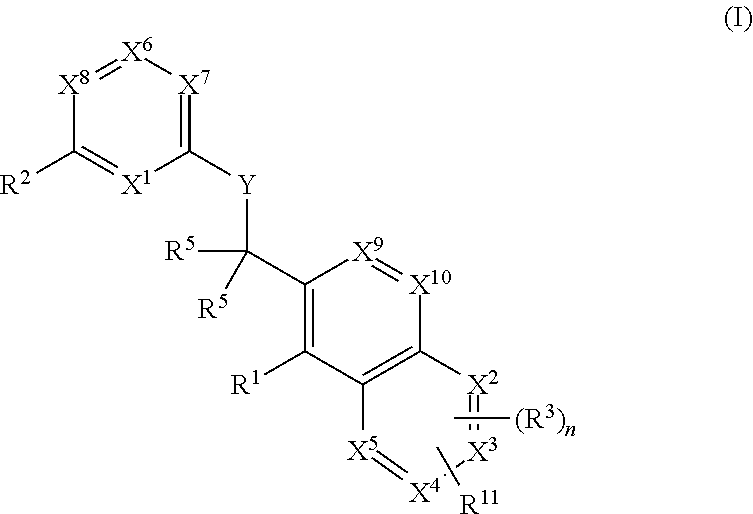
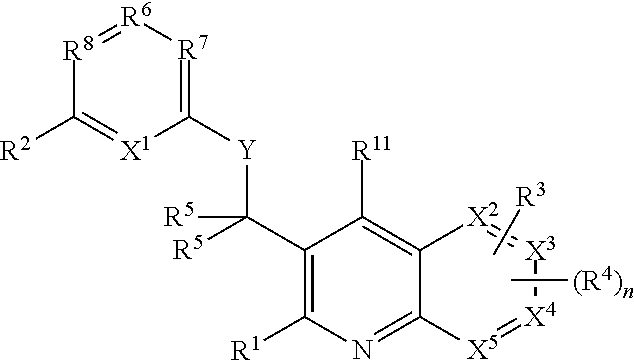
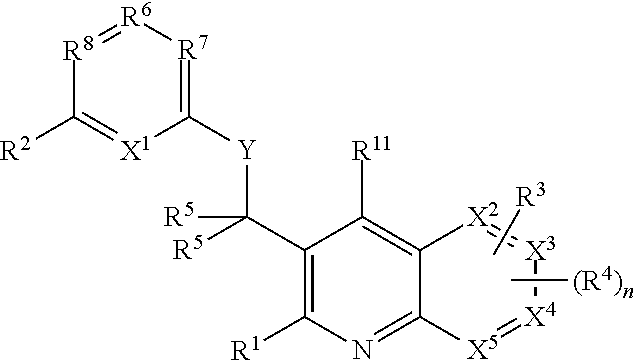
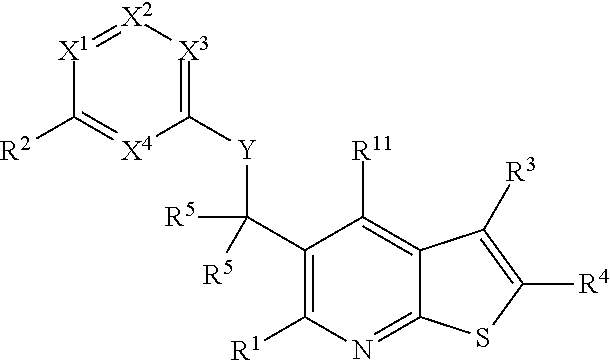
Heterocyclic compounds and their uses
Substituted bicyclic heteroaryls and compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjogren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity, The present invention also enables methods for treating cancers that are mediated, dependent on or associated with p110δ activity, including but not restricted to leukemias, such as Acute Myeloid leukaemia (AML) Myelo-dysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia (B-ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
CD19 targeting chimeric antigen receptor and NKT cell, and preparation method thereof and applications thereof
ActiveCN105418765AEnhance specific killing activityProlong survival timePeptide/protein ingredientsGenetic material ingredientsAntigenHinge region
The present invention discloses a chimeric antigen receptor, a gene and a recombinant expression vector thereof, an engineered CD19 targeting NKT cell and applications thereof. The chimeric antigen receptor is CD19ScFv-CD8-CD137-CD3 zeta, and consists of a hinge region and a transmembrane region of the CD19ScFv and CD8, an intracellular signal structural domain of CD137, and an intracellular signal structural domain of CD3 zeta, and the the hinge region, the transmembrane region, the intracellular signal structural domain of CD137 and the intracellular signal structural domain of CD3 zeta are connected in series. When used to treat advanced stage CD19-positive B-cell acute lymphocytic leukemia, the NKT cell modified by the chimeric antigen receptor CD19ScFv-CD8-CD137-CD3 zeta provided by the present invention has good specific killing activity on leukemic cells, and has certain therapeutic effect on advanced stage CD19-positive B-cell acute lymphocytic leukemia patients who are repeatedly subjected to therapy such as radiotherapy, chemotherapy and symptomatic therapy by other drugs but cannot recover obviously.
Owner:CELLULAR BIOMEDICINE GRP SHANGHAI +1
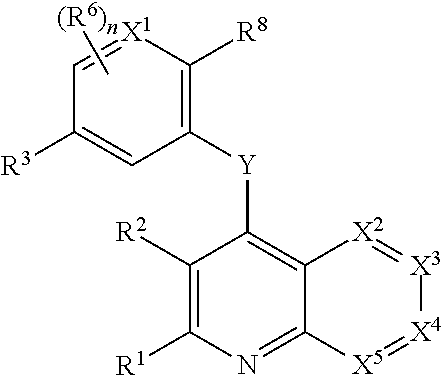
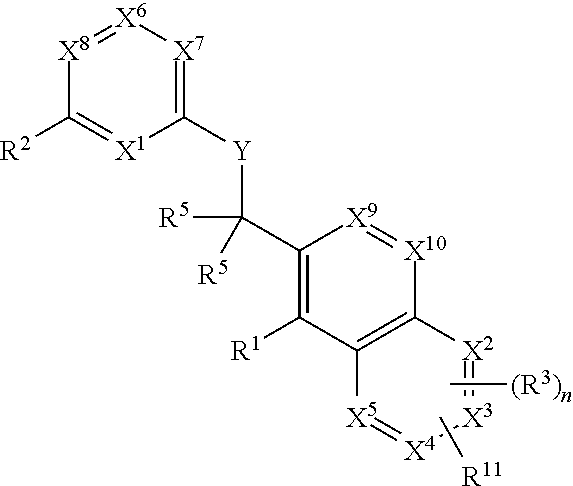
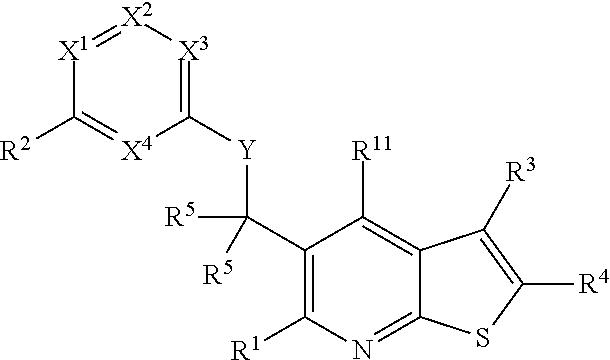
Heterocyclic compounds and their uses
Substituted bicyclic heteroaryls and compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjoegren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity. The present invention also enables methods for treating cancers that are mediated, dependent on or associated with pi 105 activity, including but not restricted to leukemias, such as Acute Myeloid leukaemia (AML) Myelo-dysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia (B-ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
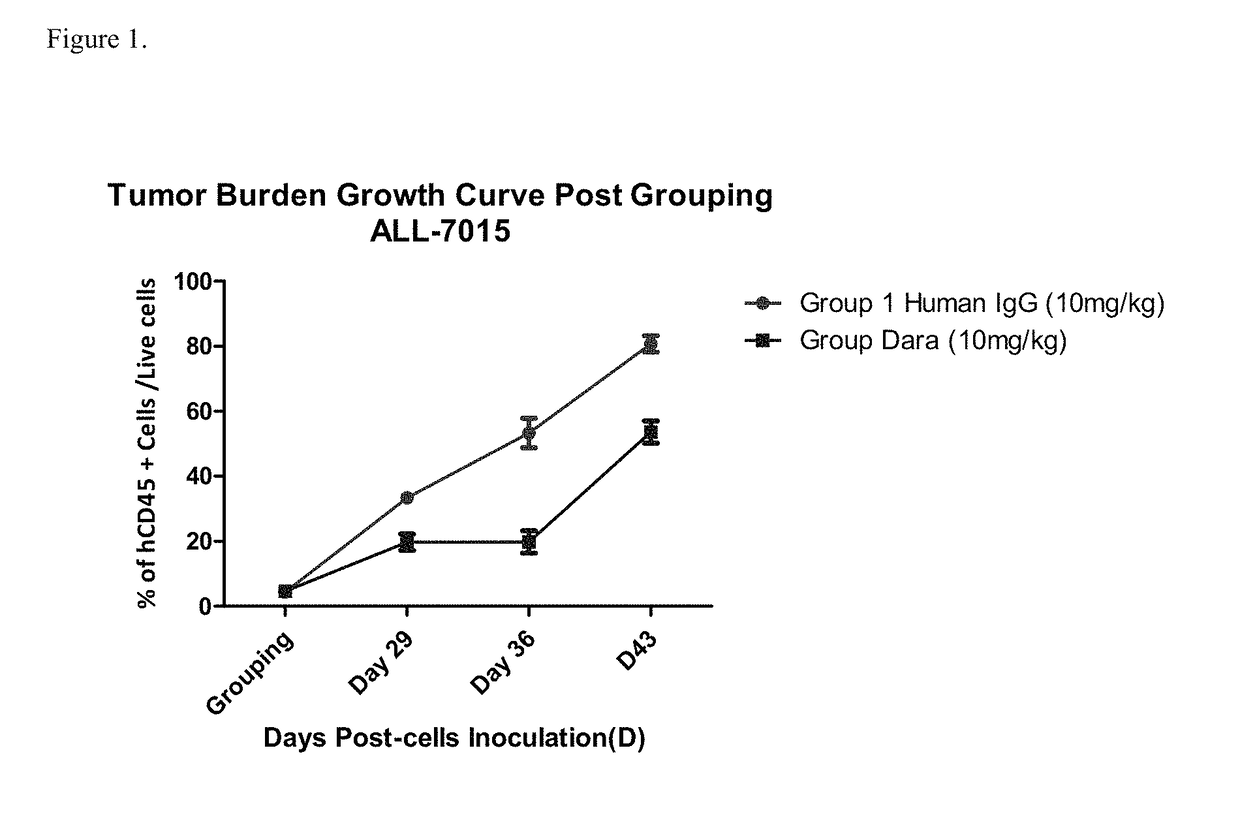
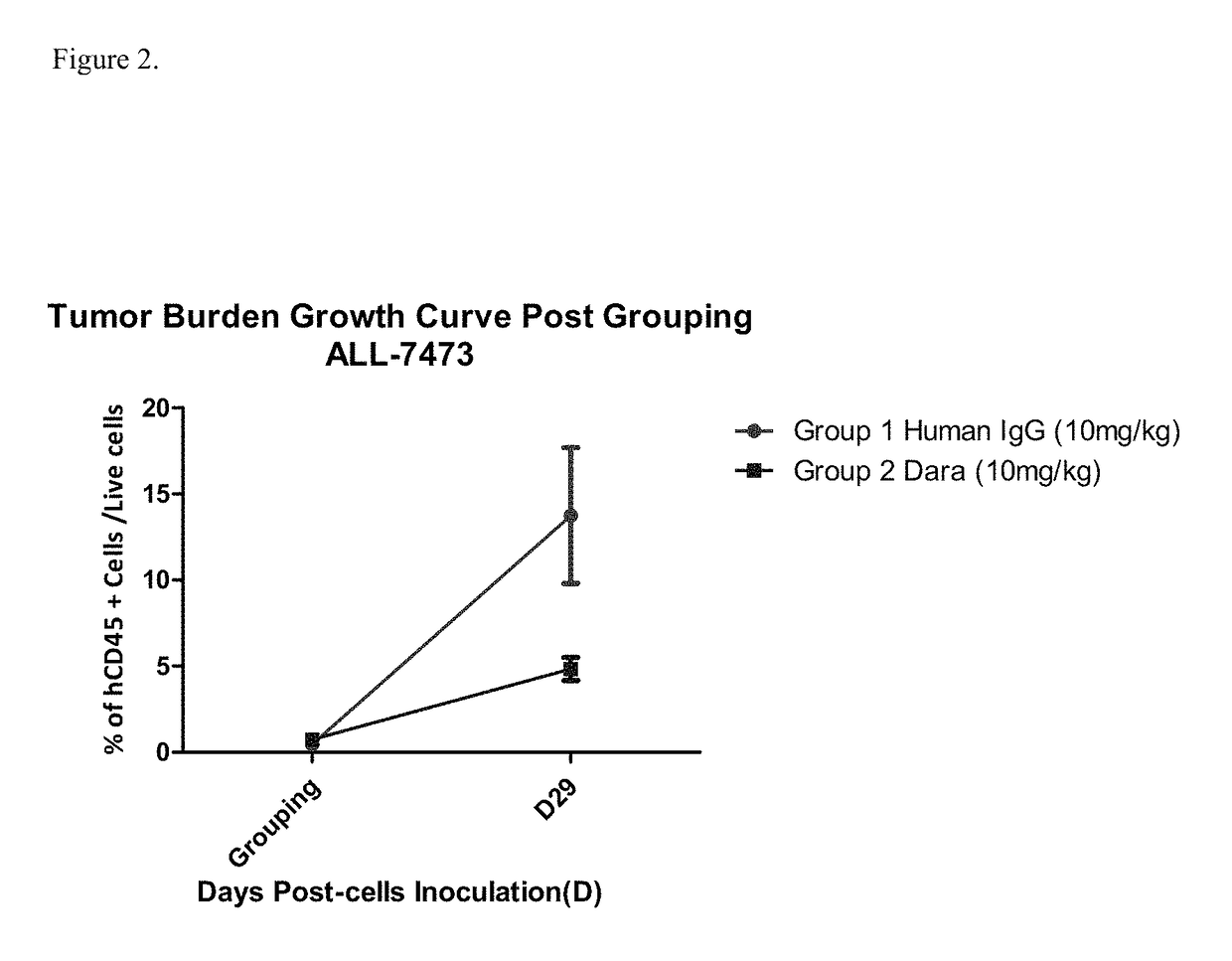
Anti-CD38 antibodies for treatment of acute lymphoblastic leukemia
ActiveUS9732154B2Organic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAcute lymphocytic leukemiaCombination therapy
Owner:JANSSEN BIOTECH INC
Highly aggressive human acute B lymphocytic leukemia cell strain with add(11)(q23) chromosome abnormality
The invention belongs to the field of microbial animal cell lines, and relates to a new human acute B lymphocytic leukemia cell strain CHH-1. The in vitro isolate of the mononuclear cell of the marrow of a patient with firstly-diagnosed acute B lymphocytic leukemia undergoes cell primary culture to obtain the highly aggressive human acute B lymphocytic leukemia cell strain with add(11)(q23) chromosome abnormality, the preservation number of the cell strain is CGMCC No.11797, and the highly aggressive human acute B lymphocytic leukemia cell strain with add(11)(q23) chromosome abnormality is named as human acute B lymphocytic leukemia cell strain CHH-1, has a same clone source with patient's leukemia cells, can be infinitely and stably passed in vitro, and has the characteristics of clonality add(11)(q23) genetic abnormality, high tumorigenicity and high aggressiveness. The new human acute B lymphocytic leukemia cell strain CHH-1 provides a new good cell model for researches of human acute lymphocytic leukemia with No.11 chromosome long arm structure abnormality and development of biomedicines, and has wide prospect and great practical values in revelation of the pathogenesis of the human acute lymphocytic leukemia and development of medicines.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
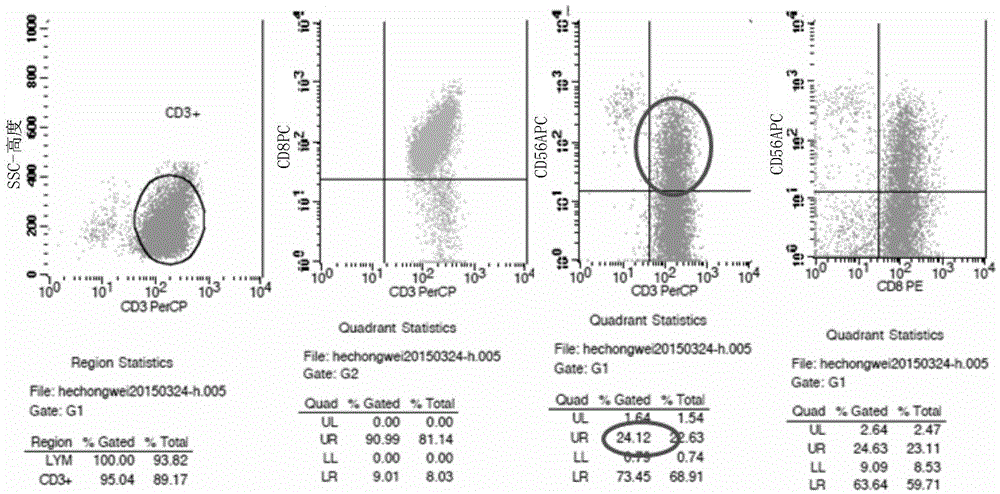
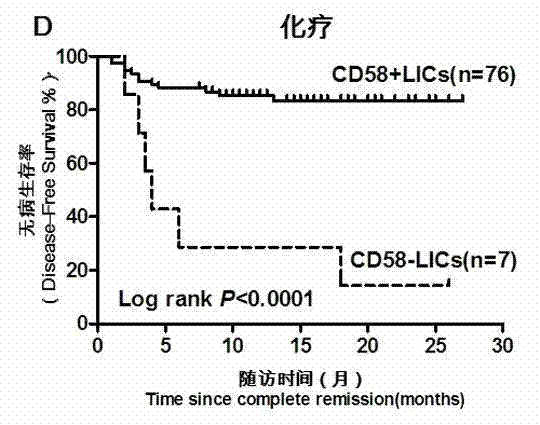
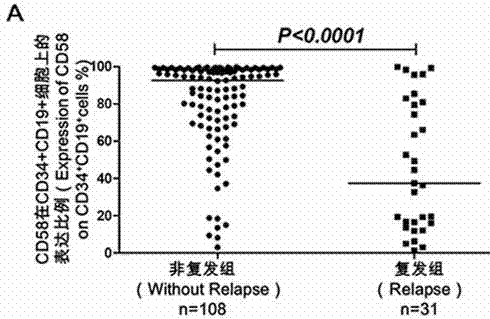
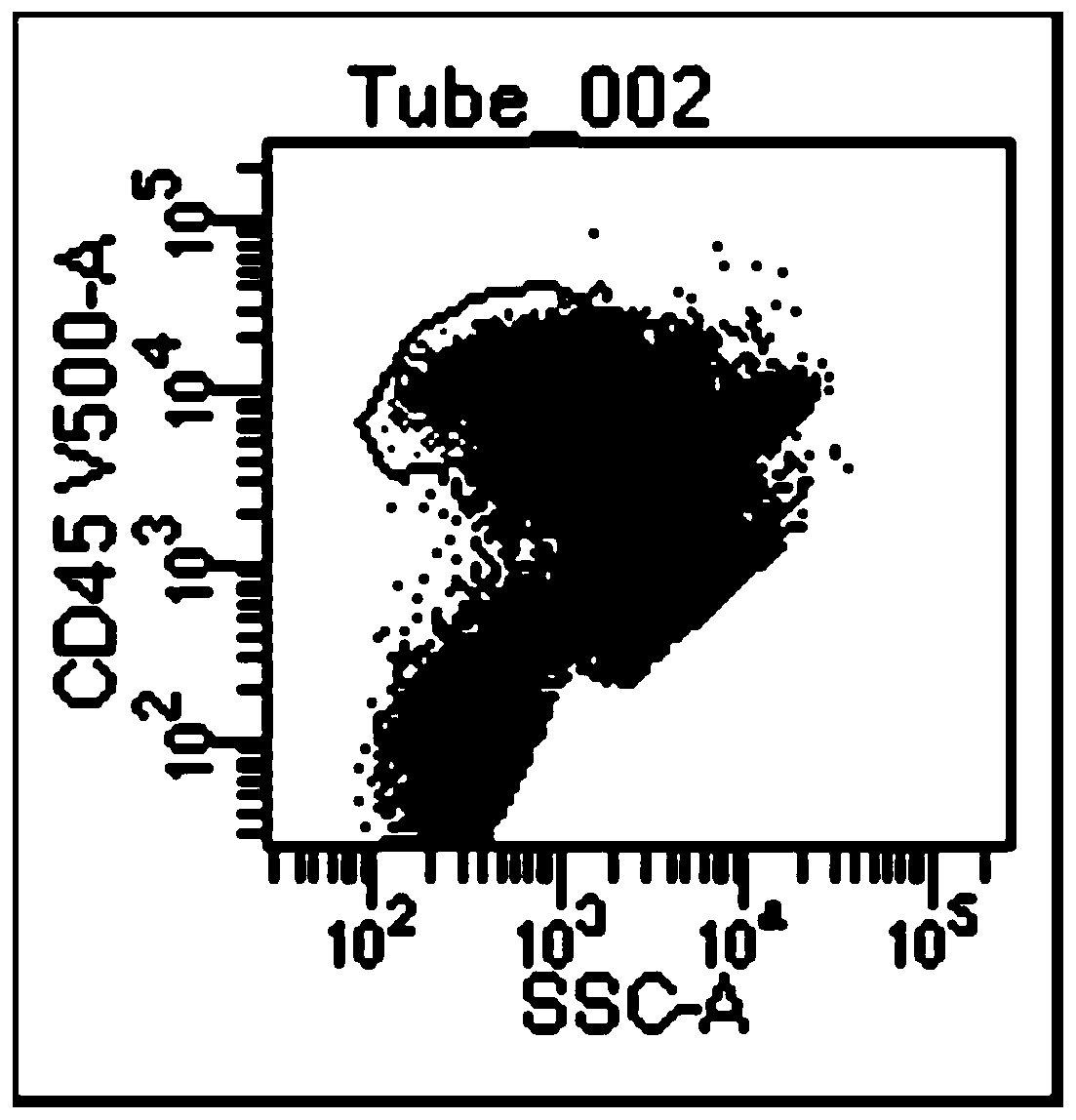
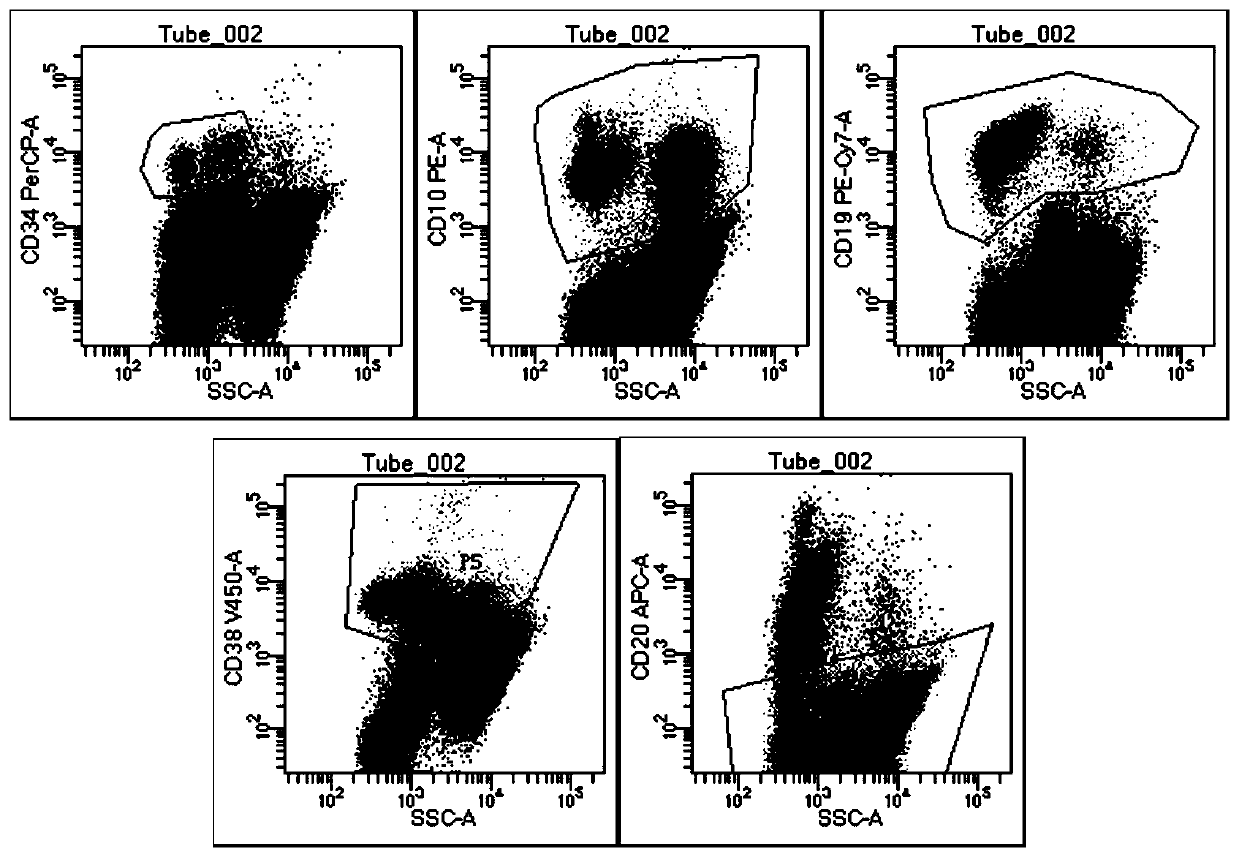
Acute B-lymphocyte leukemia initiated cell phenetic classification kit and application thereof
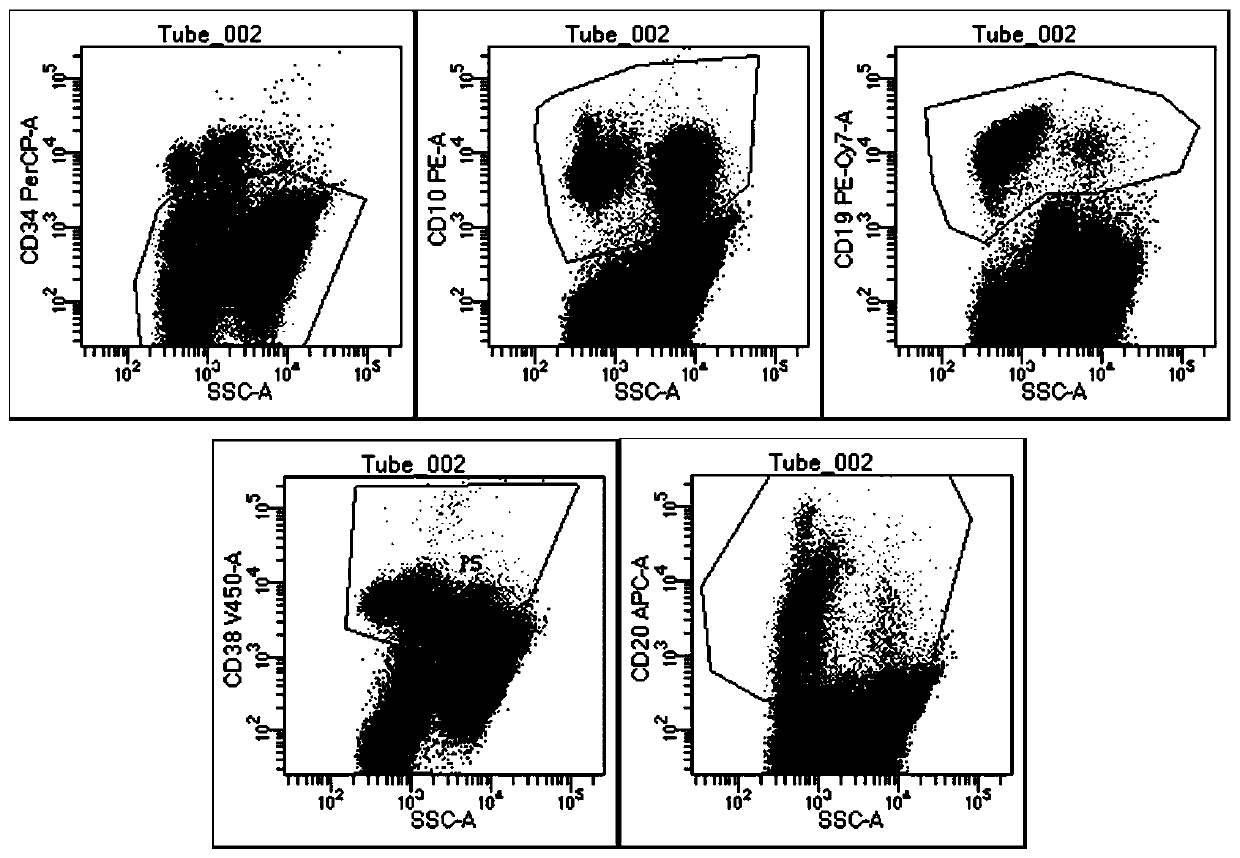
ActiveCN103175966AAccurate typingRapid TypingMaterial analysisCell phenotypeAntiendomysial antibodies
The invention discloses an acute B-lymphocyte leukemia initiated cell phenetic classification kit. The kit is used in cooperation with a sever-color flow cytometer. The structure of the kit is configured with the following fluorescently-labeled monoclonal antibody: CD58FITC, CD10PE, CD34PerCP, CD38APC, CD19APC-Cy7 and CD45PacificBlue. The kit disclosed by the invention can be used for fast and accurately detecting the immunophenotype of the leukemia initiated cell of a B-ALL patient in first visit; and the CD58 expression proportion on the leukemia initiated cell tested by the kit provided by the invention can be used as one of the prognostic indicators of the B-ALL patient, and the indicator has important guiding significance in the prognosis of the B-ALL patient and the decision of a clinic treatment scheme.
Owner:PEOPLES HOSPITAL PEKING UNIV
Method for constructing humanized Ph chromosome positive acute lymphocytic leukemia mouse model
InactiveCN103740639AImprove efficiencyGood repeatabilitySkeletal/connective tissue cellsAnimal husbandryAcute lymphoblastic leukemiasMouse monoclonal antibody
The invention discloses a method for constructing humanized Ph chromosome positive acute lymphocytic leukemia mouse model, and the method comprises the following steps: acquisition of leukemia cells, pretreatment of NOD / SCID mouse and implantation of leukemia cells, and implantation of leukemia cells. Specifically, the method comprises: 60Co irradiation of sub-lethal dose combined with a pretreatment with rat anti-mouse CD122 monoclonal antibody, and injection of Ph+ALL bone marrow mononuclear cells from knee joint marrow cavity of NOD / SCID mouse, thereby a humanized Ph+ALL mouse model is established with high molding efficiency and good repeatability; cell sorting is not needed, and the molding process is easy to be operated; the method provides an experiment platform for carrying out mankind Ph+ALL research on the level of living animals, and will promote deep research of domestic Ph+ALL.
Owner:PEOPLES HOSPITAL PEKING UNIV
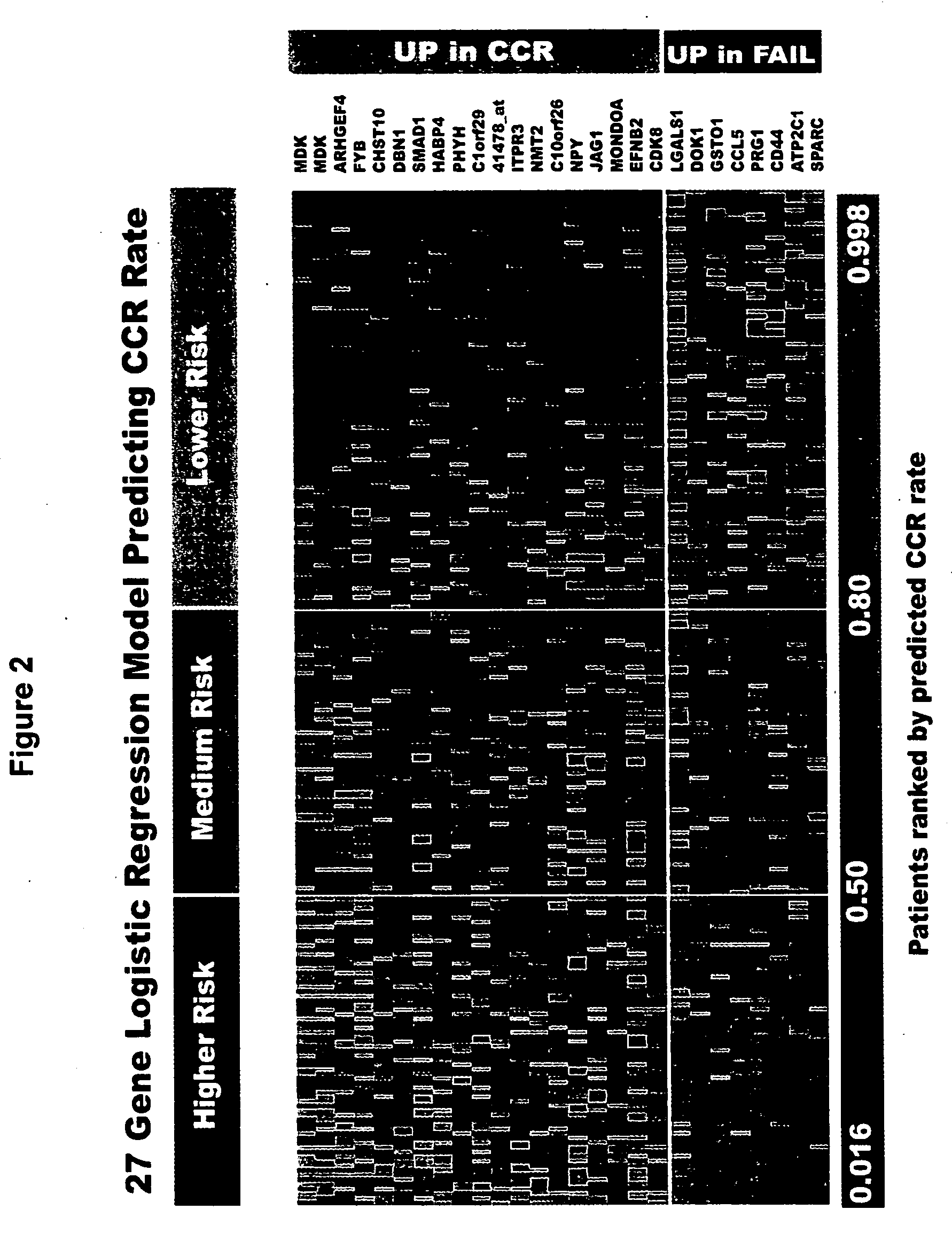
Molecular technologies for improved risk classification and therapy for acute lymphoblastic leukemia in children and adults
InactiveUS20060141504A1Long durationMicrobiological testing/measurementTherapy OutcomeRisk classification
The present invention relates to methods for predicting the outcome of therapeutic intervention in cases of leukemia, especially acute lymphoblastic leukemia in children and adults. The present invention evaluates a gene expression profile and identifies prognostic genes of cancers, in particular leukemia, more particularly B-precursor acute lymphoblastic leukemia (ALL). The present invention provides a method of determining prognosis of leukemia, in particular, acute lymphoblastic leukemia, more particularly B-precursor ALL and predicting therapeutic outcome of a patient. The method comprises the steps of first establishing the threshold value of at least three prognostic genes of leukemia, preferably at least eight prognostic genes, or preferably, as many as 26 prognostic genes. Then, the amount of the prognostic gene(s) from a leukemia patient is determined. The amount of the prognostic gene present in that patient is compared with the established threshold value of the prognostic gene(s) which is indicative of therapeutic success or failure, whereby the prognostic outcome of the patient is determined / predicted.
Owner:STC UNM
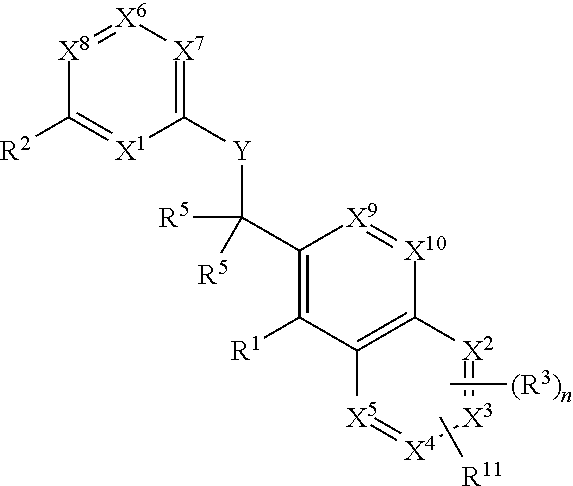
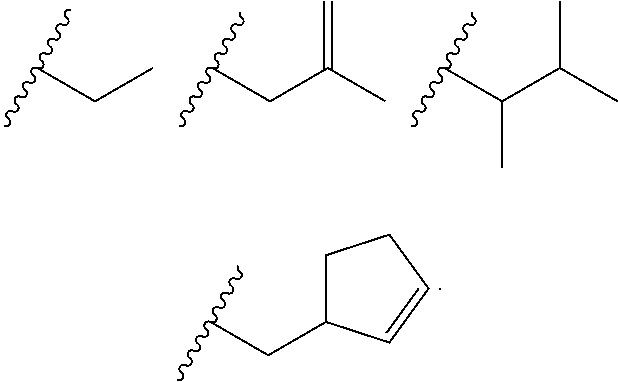
Heterocyclic compounds and their uses
Substituted bicyclic heteroaryls and compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjoegren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity, The present invention also enables methods for treating cancers that are mediated, dependent on or associated with pi 105 activity, including but not restricted to leukemias, such as Acute Myeloid leukaemia (AML) Myelodysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia (B-ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
Detection kit for minute residues of B-cell acute lymphocyte leukemia
InactiveCN109884313AImprove the detection rateReduce missed detection rateBiological testingCD20Leukocyte Differentiation
The invention relates to the technical field of medical detection, in particular to a detection kit for minute residues of B-cell acute lymphocyte leukemia. The detection kit comprises anti CD10, CD19, CD20, CD34, CD38 and CD45 antibodies labeled by different fluoresceins. The detection kit comprises six monoclonal antibody combinations of leukocyte differentiation antigen, and a fluorescence labeling and flow cytometric detection technology is combined to realize that one-time flow cytometric detection can quickly and accurately identify the level and typing of abnormal cells of the minute residues of the B-cell acute lymphocyte leukemia, the obtained detection index can be used as an intermediate result to intuitively judge the prognosis conditions of B-cell acute lymphocyte leukemia patients, and thus important guiding significance is provided for the formulation of clinical treatment schemes for the leukemia patients.
Owner:武汉康圣达医学检验所有限公司
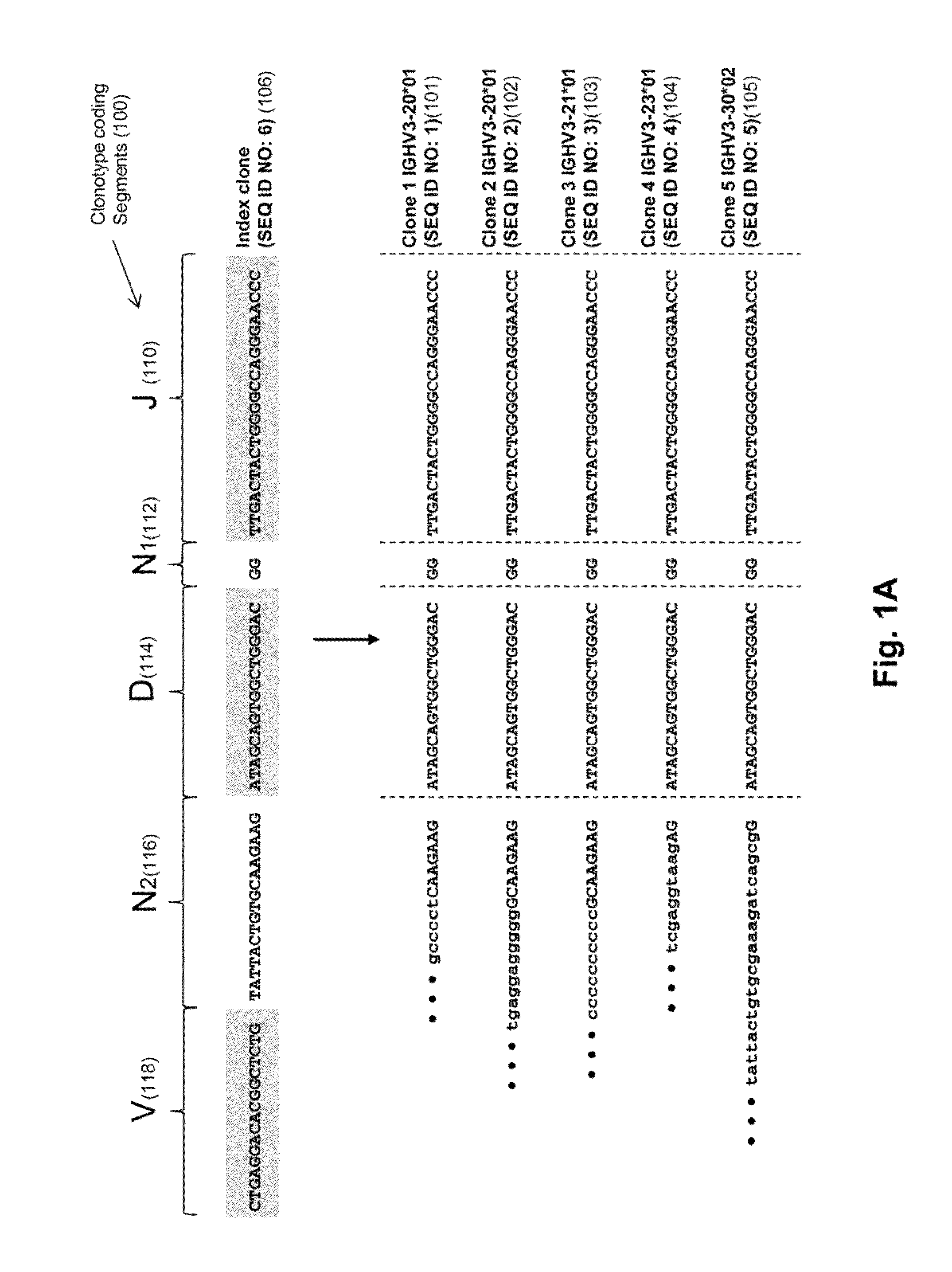
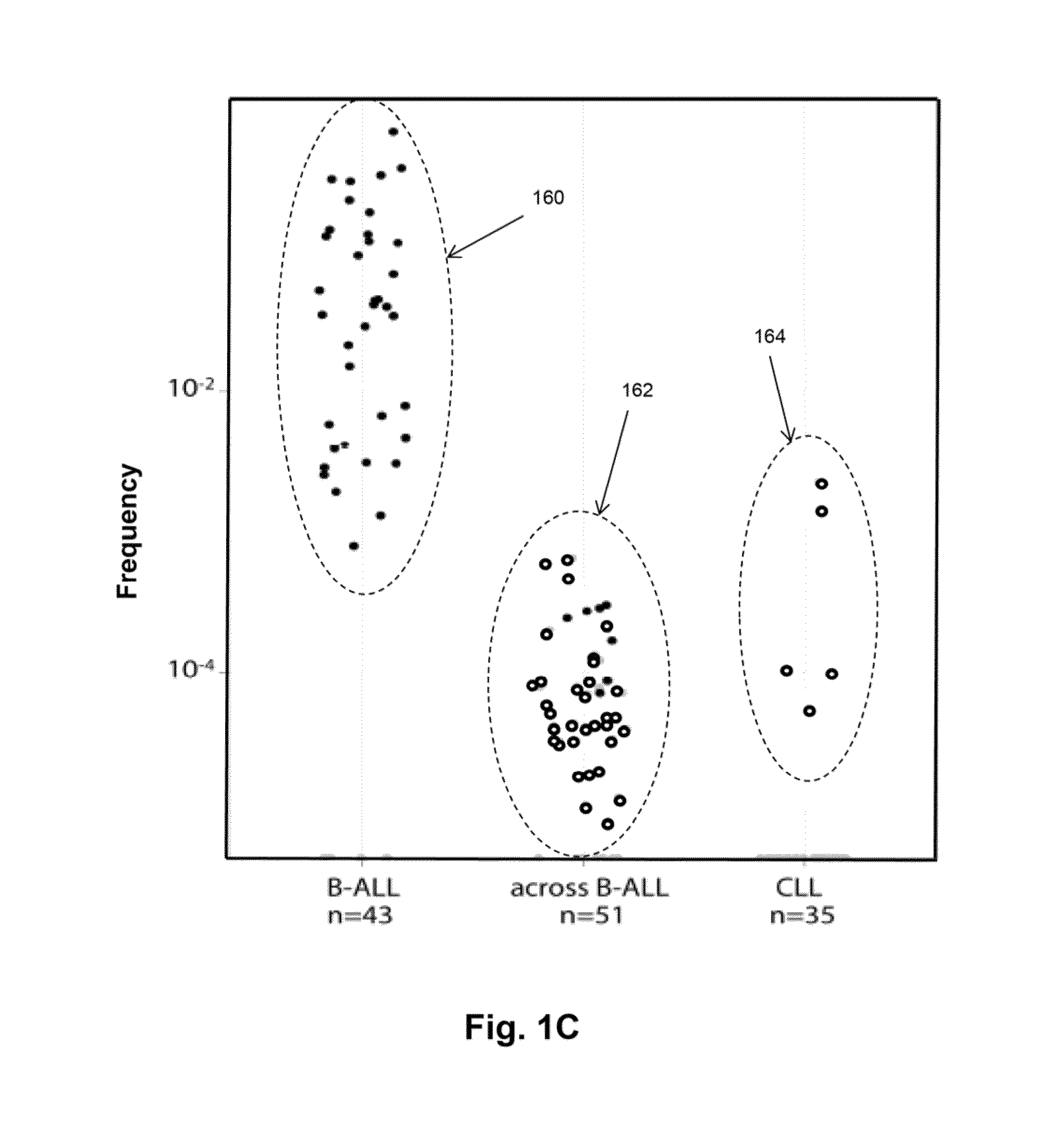
Monitoring immunoglobulin heavy chain evolution in B-cell acute lymphoblastic leukemia
ActiveUS9365901B2Reduce the possibilityOvercome deficienciesHeavy metal active ingredientsMicrobiological testing/measurementImmunoglobulin heavy chainDisease
The invention is directed to methods of monitoring B-cell lymphoid proliferative disorders, such as B-cell acute lymphoblastic leukemias, by measuring the presence, absence and / or levels of correlating, or index, clonotypes and related clonotypes that have evolved therefrom, for example, as part of the disease condition. In one aspect, such methods are implemented by generating sequencing-based clonotype profiles and determining frequencies of correlating, or index, clonotypes present, including new clonotypes that have evolved therefrom, particularly, in the case of B-cell ALL, by VH substitution. The invention also includes use of such monitoring information to modify treatment status of a patient.
Owner:ADAPTIVE BIOTECH
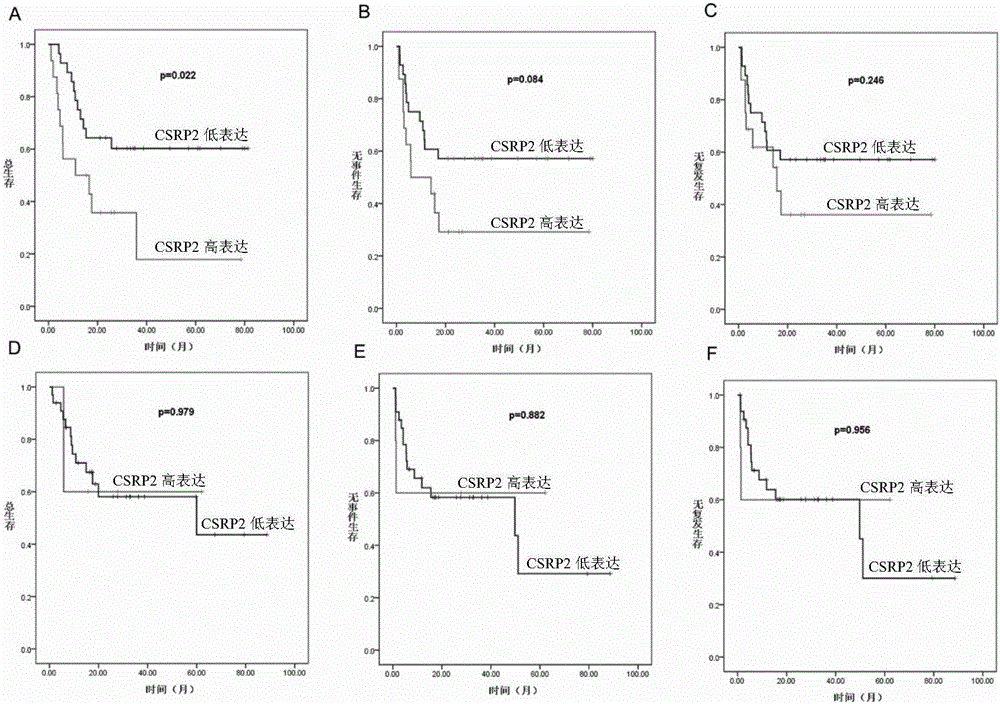
Application of CSRP2 serving as marker for evaluating prognostic risk of adult B-ALL patients
The invention discloses application of CSRP2 serving as a marker for evaluating prognostic risk of adult B-ALL patients. The application provided by the invention is particularly the application of a detection substance for detecting mRNA coding CSRP2 protein in preparation of a product for evaluating the prognostic risk of patients suffering acute b lymphoblastic leukemia or application in evaluation of the prognostic risk of the patients suffering acute b lymphoblastic leukemia. Experimental results prove that CSRP2 low expression may be the independent risk factor of the OS, EFS and RFS of an adult B-ALL patient and may be play important roles in hierarchical diagnosis and prognostic evaluation of B-ALL.
Owner:北京旌准医疗科技有限公司
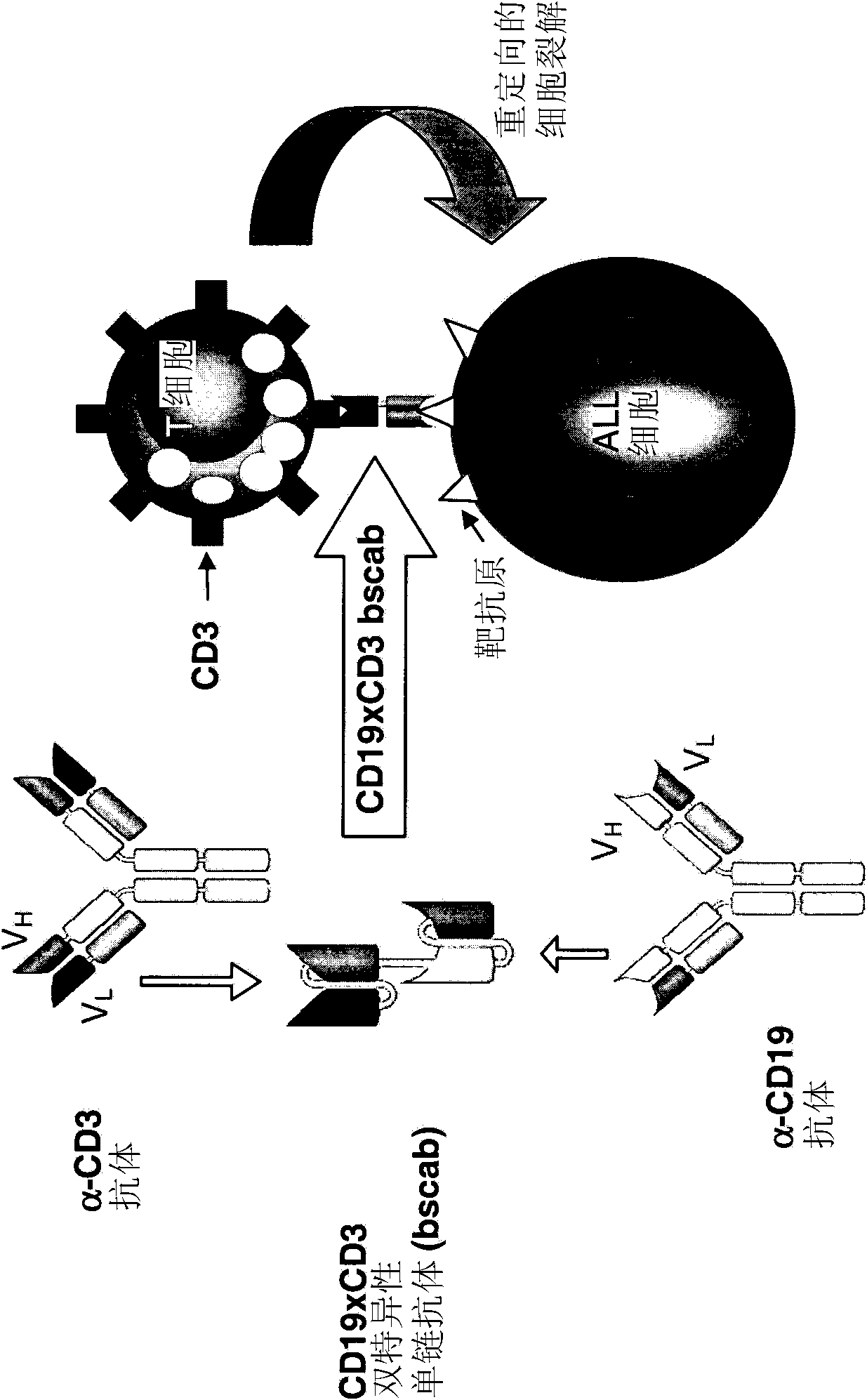
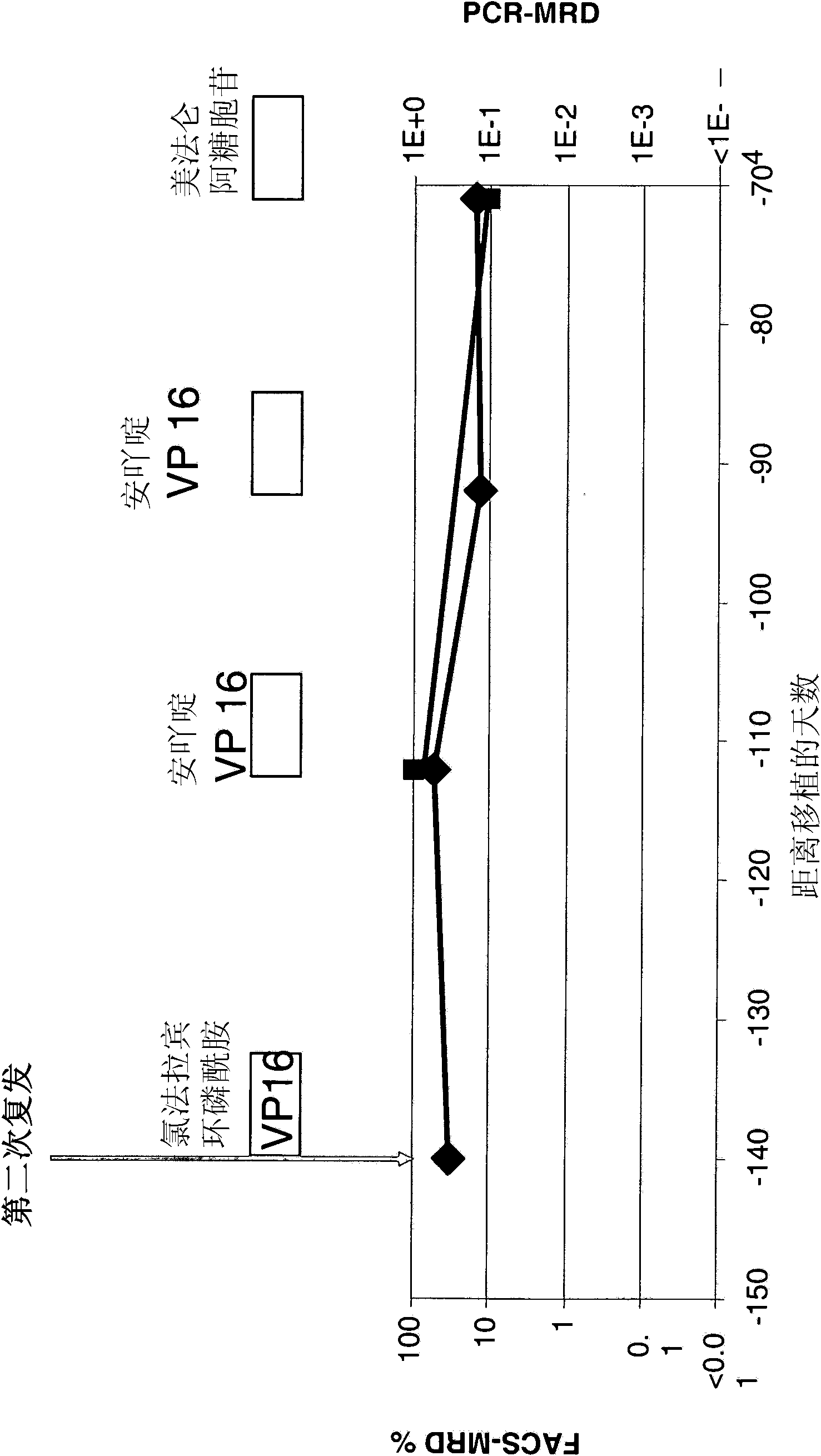
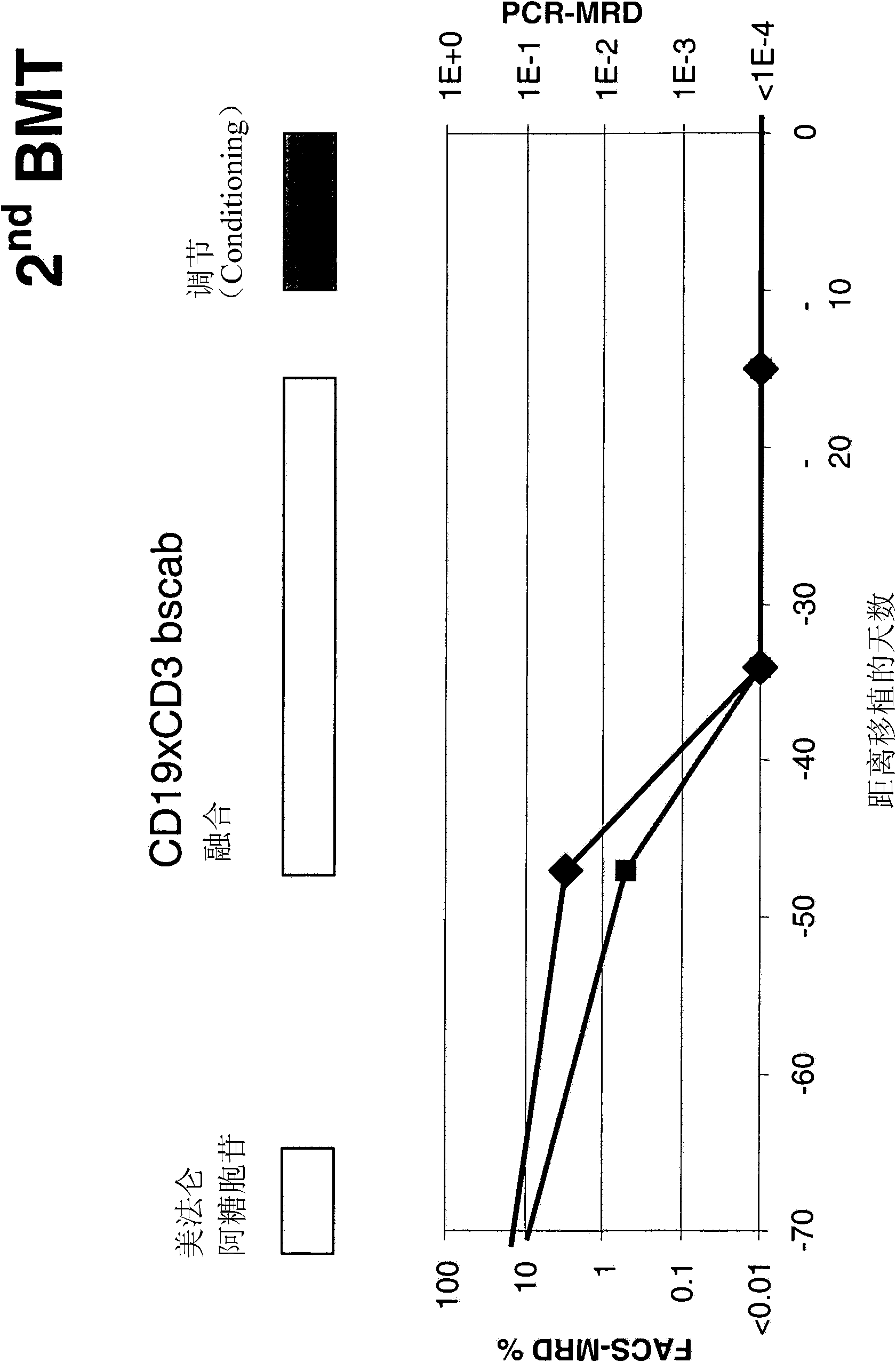
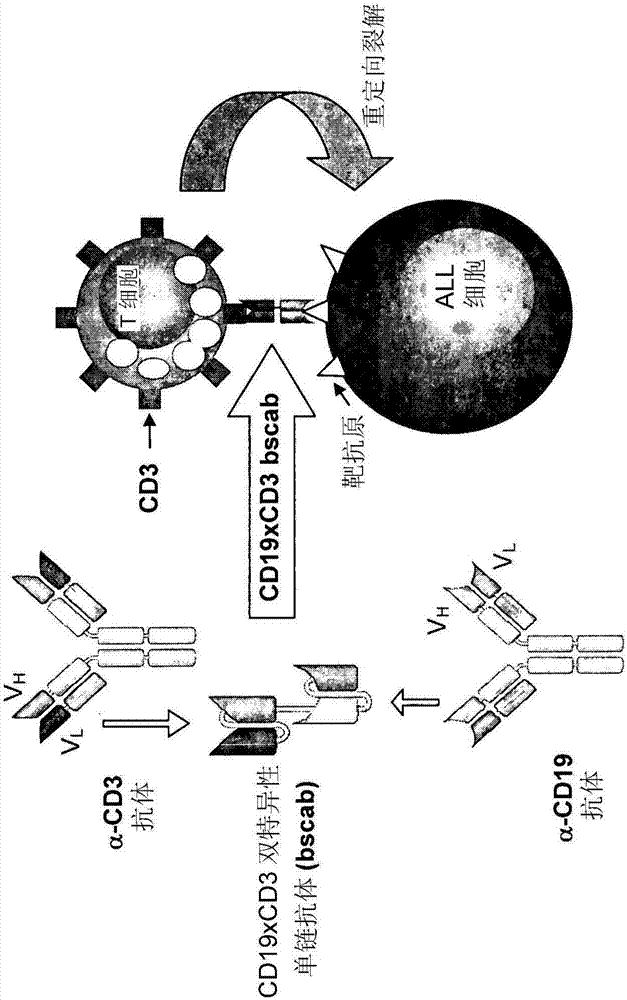
Treatment of pediatric acute lymphoblastic leukemia
ActiveCN102209729AEliminate the risk of recurrenceEffective anti-leukemia effectHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsDrugSingle-Chain Antibodies
The present invention relates to a method for the treatment, amelioration or elimination of pediatric acute lymphoblastic leukemia (ALL), the method comprising the administration of a pharmaceutical composition comprising a CD19xCD3 bispecific single chain antibody construct to a pediatric ALL patient in the need thereof.
Owner:AMGEN RES (MUNICH) GMBH
Tubulin depolymerizing agent polypeptides and application thereof
InactiveCN103254287AInhibition of aggregation activityImprove survival ratePeptide/protein ingredientsPeptidesChemical synthesisMedicine
The invention relates to tubulin depolymerizing agent polypeptides and application thereof, particularly polypeptides capable of inhibiting polymerization of tubulin and treating acute lymphatic leukemia. The invention is characterized in that the sequence of the tubulin depolymerizing agent polypeptides 1 is CRALERLV disclosed as SEQ NO.1. The invention also relates to application of the tubulin depolymerizing agent polypeptides 1 in preparation of medicines for treating acute lymphatic leukemia. A solid-phase synthesis method is utilized to chemically synthesize the tubulin depolymerizing agent polypeptides 1; and the polypeptides with the bran-new sequence can inhibit tubulin in vitro and treat acute lymphatic leukemia.
Owner:河北佑仁生物科技有限公司
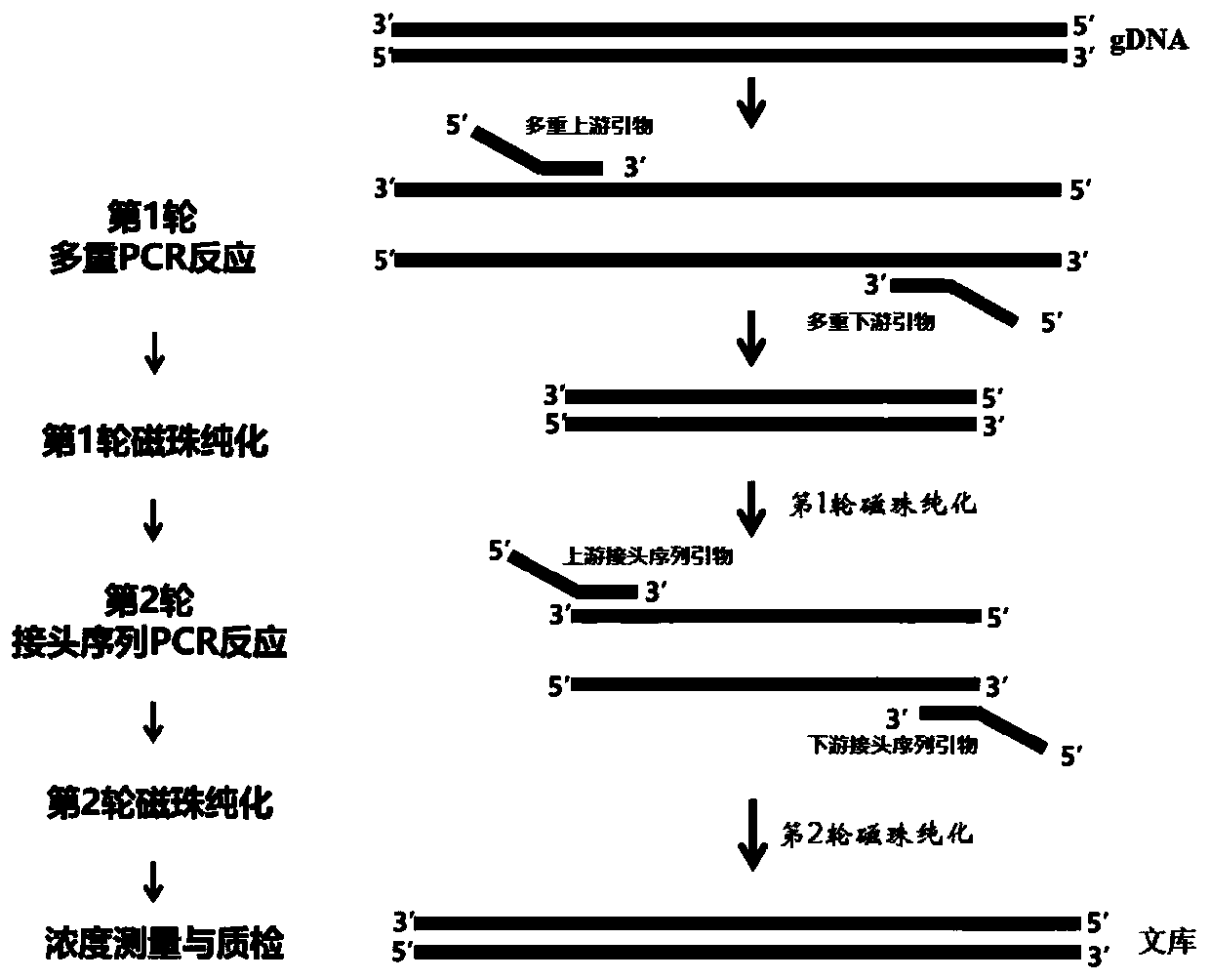
Kit and method for detecting gene mutation of acute lymphoblastic leukemia based on multiplex-PCR targeted high-throughput sequencing
PendingCN111575373AImprove accuracyIncrease coverageMicrobiological testing/measurementDNA/RNA fragmentationMultiplexGenes mutation
The invention discloses a kit for detecting gene mutation of acute lymphoblastic leukemia based on multiplex-PCR targeted high-throughput sequencing and a detection method of the kit. The kit is usedfor detecting multiple exon regions of 19 genes related with the acute lymphoblastic leukemia. The kit contains a primer working solution for detecting relevant gene mutation, a PCR mixed solution, aPCR reaction solution, a digestion solution, a joint P5 and a joint P7. The kit is used for carrying out detection based on a multiplex-PCR targeted high-throughput sequencing technique, has the advantages of high accuracy, sensitivity and throughput and can be used for rapidly and efficiently carrying out standardized quantitative detection on 19 relevant genes of acute lymphoblastic leukemia (ALL).
Owner:南京实践医学检验有限公司
Heterocyclic compounds and their uses
Substituted bicyclic heteroaryls and compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjoegren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity, The present invention also enables methods for treating cancers that are mediated, dependent on or associated with p110 activity, including but not restricted to leukemias, such as Acute Myeloid leukaemia (AML) Myelo-dysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia (B-ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
Mouse model of acute lymphoblastic leukemia and modeling method
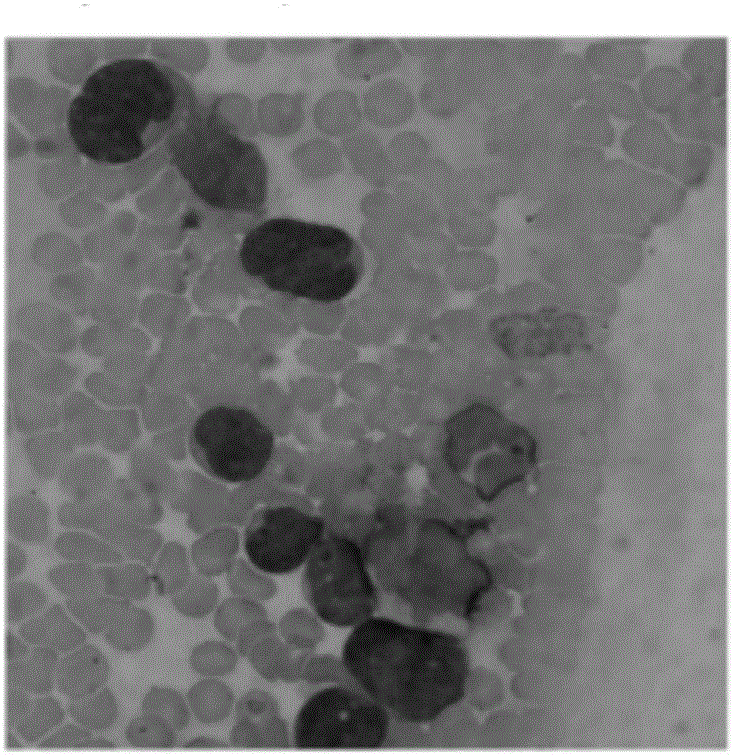
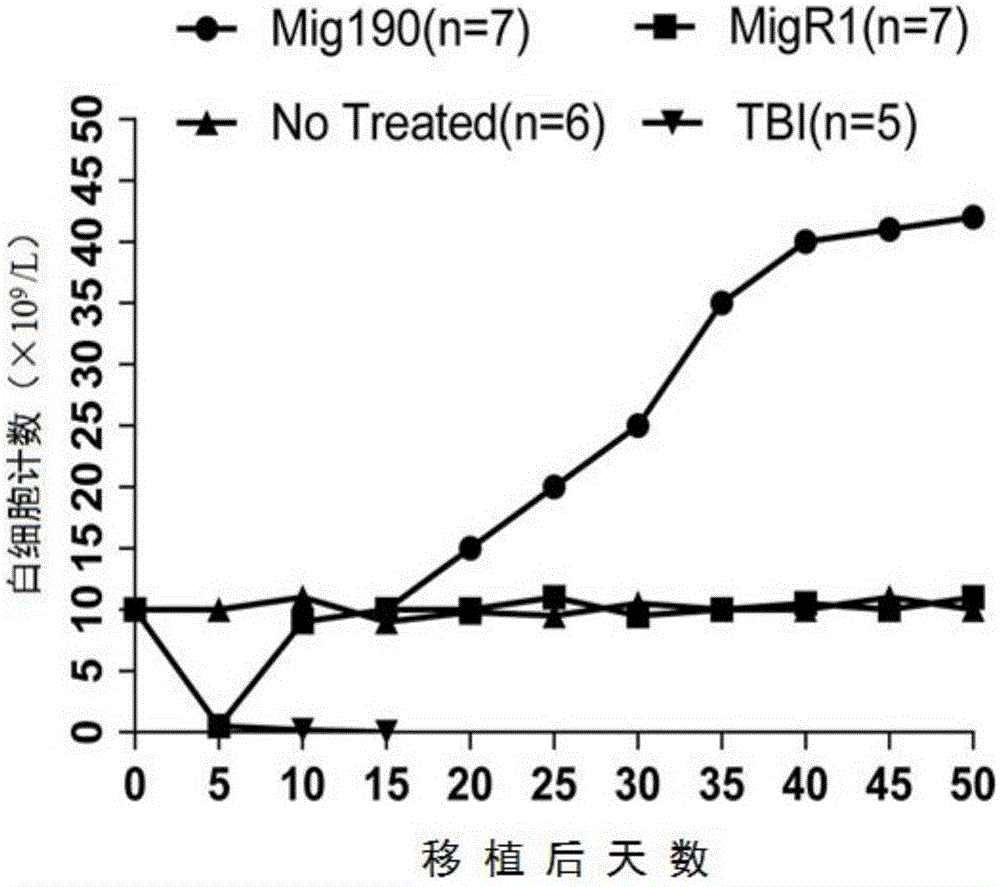
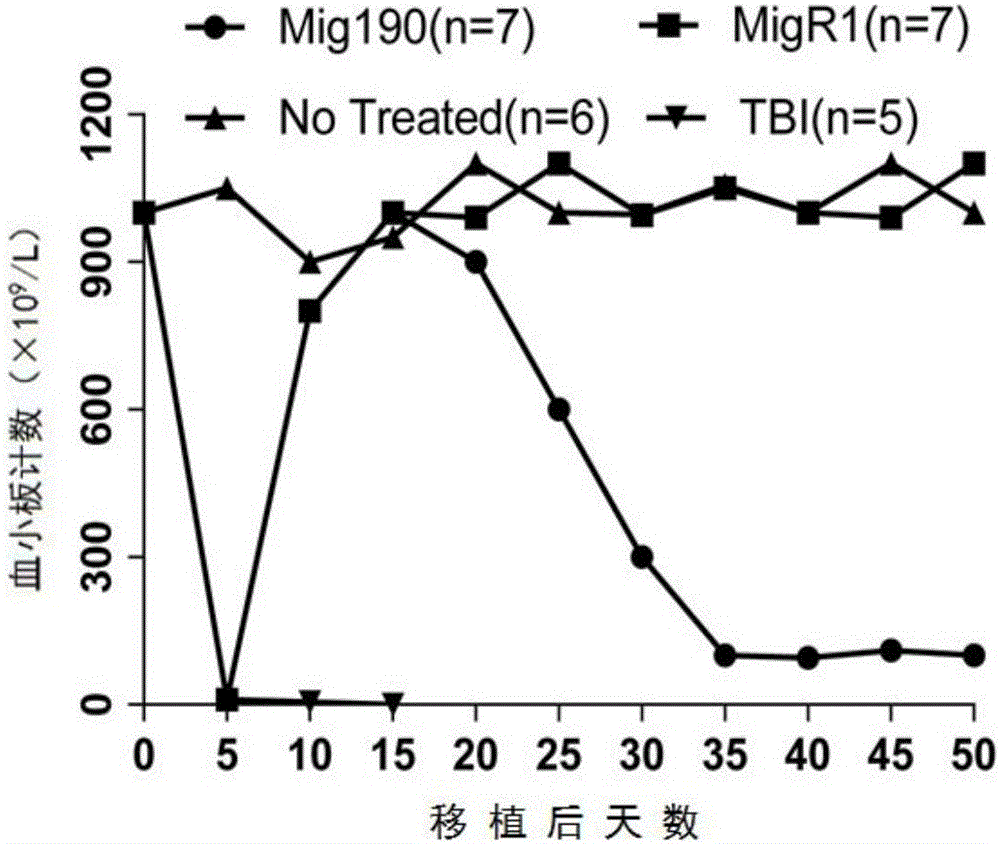
ActiveCN106520805ASimplify the modeling processReduce the risk of accidental deathBlood/immune system cellsFermentationHematopoietic cellAcute lymphocytic leukemia
The invention provides a mouse model of acute lymphoblastic leukemia and a modeling method, and relates to the field of biotechnology. The preparation method comprises the following steps: infecting IL-7 stimulated hematopoietic cells with retroviruses with BCR-ABLP190; and transfusing the stimulated hematopoietic cells to syngenic mice. The method has the advantages of being simple and easy to implement in a modeling process and capable of establishing the ph+ALL mouse model in one step. According to the mouse model of the acute lymphoblastic leukemia provided by the invention, the sorted BCR-ABL+B cells are transfused to the syngenic mice for the second time without irradiation treatment, with performance consistent with that of mice of first episode; the model is good in stability; and in addition, the uniformity of recipient mice is enhanced and such risks as accidental death of the recipient mice due to irradiation are reduced.
Owner:XUZHOU MEDICAL UNIV
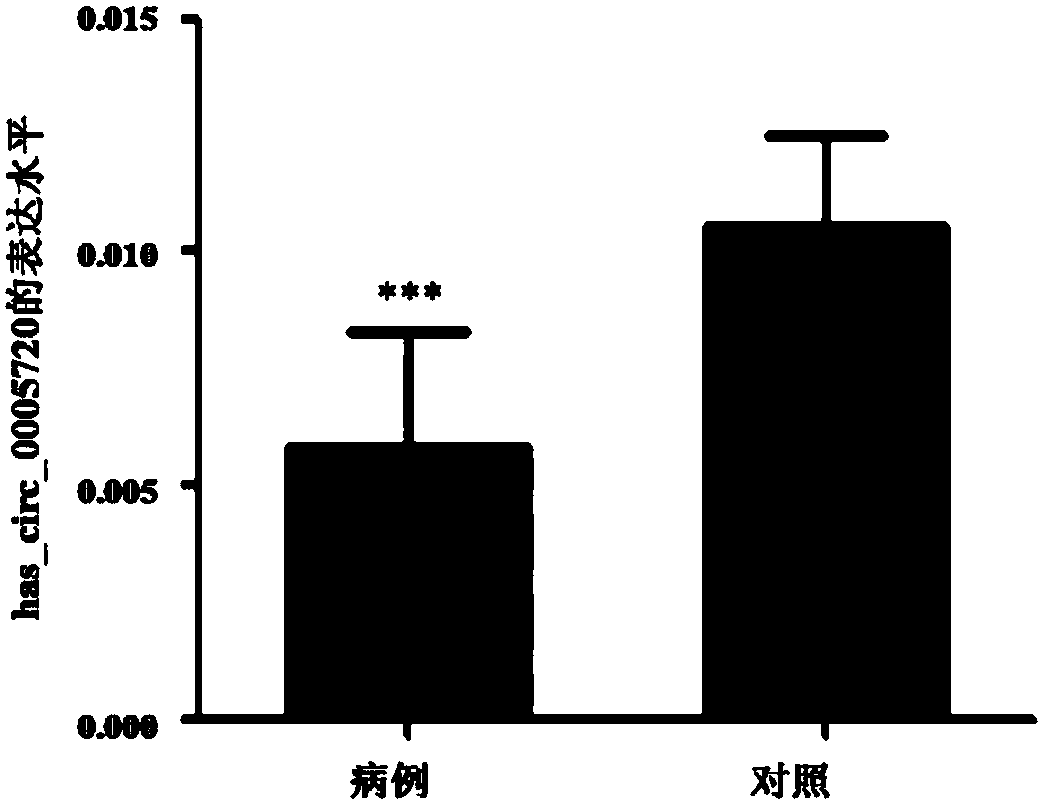
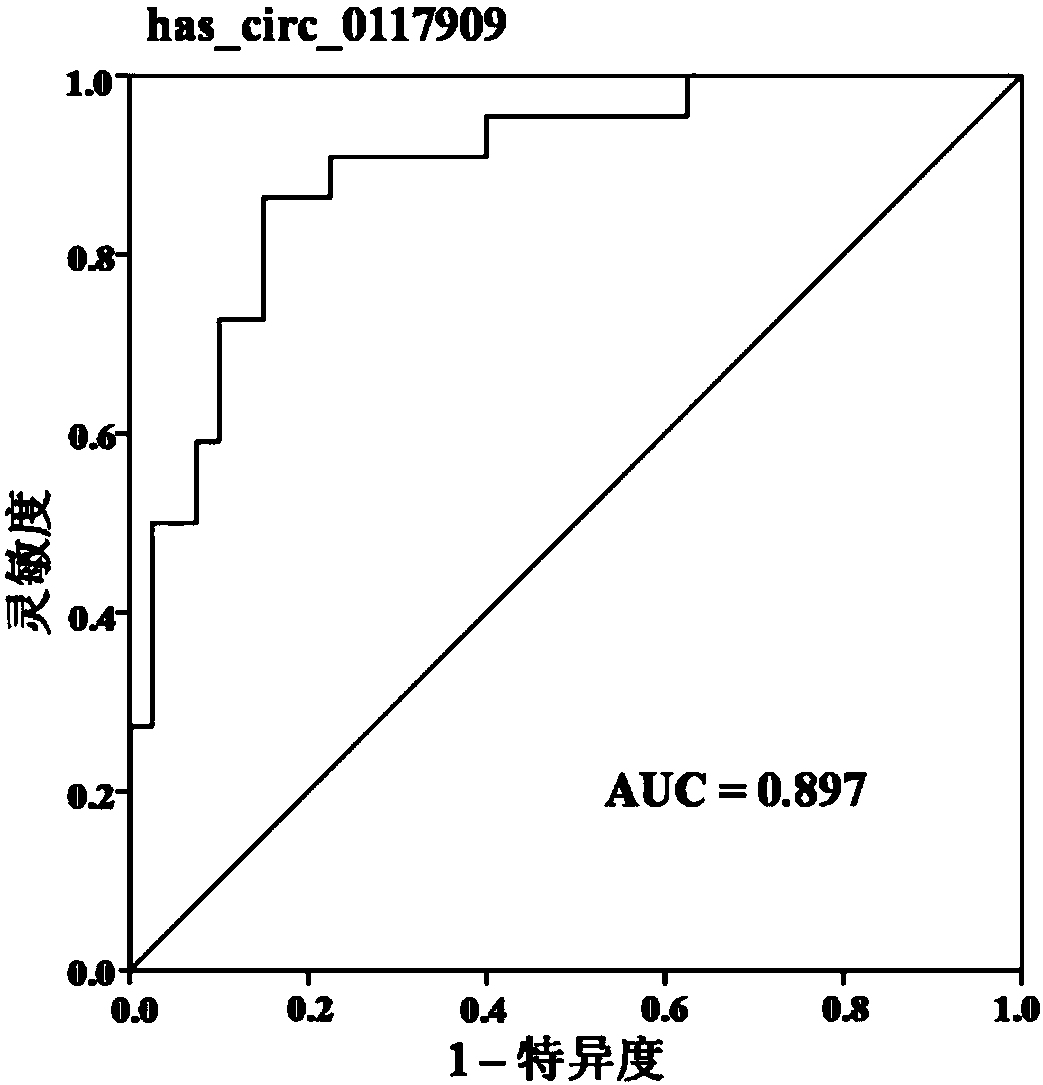
Group of circular RNA (circRNA) markers for diagnosis of childhood acute lymphoblastic leukemia (ALL) and application of group of circRNA markers for diagnosis of childhood ALL
ActiveCN107937522AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationChildhood allBone marrow
The invention discloses a group of circRNA markers for diagnosis of childhood ALL and application of the group of the circRNA markers for the diagnosis of childhood ALL. The markers comprise two circRNAs which are has_circ_0117909 as shown in SEQ ID No: 1 and has_circ_0005720 as shown in SEQ ID No: 2. The circRNA biomarkers for diagnosis of childhood ALL provided by the invention can be used forthe diagnosis of the childhood ALL, AUC of the circRNA biomarkers for distinguishing bone marrow of childhood ALL from normal control bone marrow can reach 0.962, the sensitivity reaches 90.9%, and the specificity reaches 95%.
Owner:NANJING CHILDRENS HOSPITAL
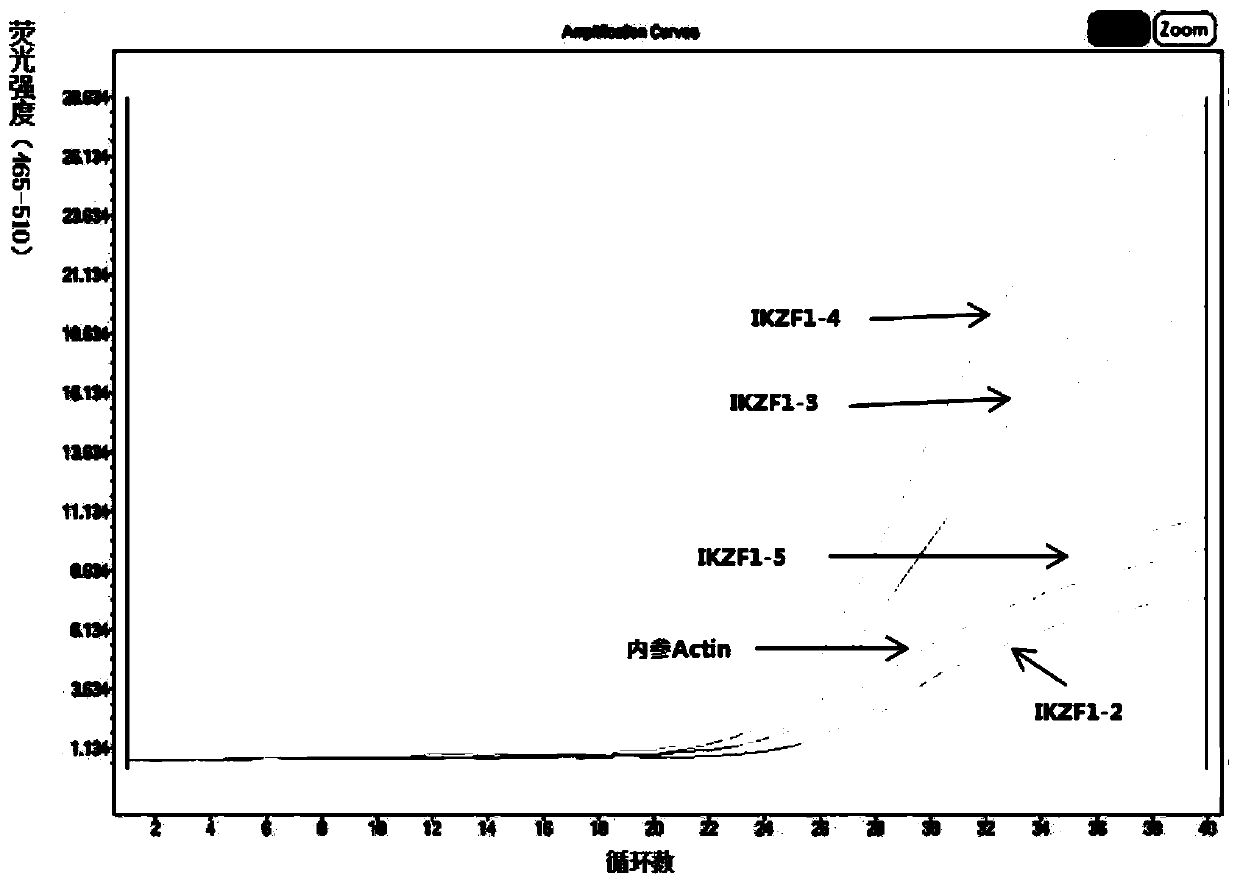
Kit for detecting gene mutations in acute lymphoblastic leukemia based on fluorescence quantitative PCR and method
PendingCN111534588ASimple and fast operationHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationNucleotideMedicine
The invention discloses a kit for detecting gene mutations in acute lymphoblastic leukemia based on fluorescence quantitative PCR. The kit comprises a PCR primer pair, a probe, a 2x PCR Nucleotide Mixreaction solution and RNase-Free ddH20. The kit has the advantages that a fluorescence quantitative PCR method is adopted to carry out specific amplification detection on PAX5 and IKZF1 gene mutationregion sequences, while high sensitivity and specificity are ensured, more mutation sites are considered. The detection kit provided by the embodiment is high in sensitivity, good in specificity andsimple to operate, and is a human PAX5 and IKZF1 gene mutation detection kit with excellent performance.
Owner:南京实践医学检验有限公司
Treatment of acute lymphoblastic leukemia
PendingCN107184977AAmeliorating or palliative treatment pathwaysReduce or even eliminate the risk of recurrenceAntibody ingredientsImmunoglobulinsSingle-Chain AntibodiesLymphocytic cell
The present invention relates to a method for the treatment, amelioration or elimination of acute lymphoblastic leukemia (ALL), the method comprising the administration of a pharmaceutical composition comprising a CD19xCD3 bispecific single chain antibody construct to an adult patient in the need thereof.
Owner:AMGEN RES (MUNICH) GMBH
N-terminal polypeptide of retinoic acid induced protein 16 and preparation method and application of antibody thereof
InactiveCN103102392AExcellent hydrophilic structureExcellent flexibility zoneSerum immunoglobulinsImmunoglobulins against animals/humansAntigenLung cancer
The invention relates to the technical field of biomedicine and provides an N-terminal polypeptide of retinoic acid induced protein 16 (RAI16). The N-terminal polypeptide has an amino acid sequence represented by SEQ ID NO: 1 and excellent hydrophylic structures, flexible regions, antigen indexes and surface probability structures. The invention further provides an antibody of the N-terminal polypeptide of the RAI16 and a preparation method of the antibody. The antibody of N-terminal polypeptide of the RAI16, prepared by the invention, is high in valence and good in specificity and can generate a specific conjugation reaction with natural human RAI16 molecules. Furthermore, the invention provides applications of RAI16 N-terminal polypeptide and the antibody thereof in the preparation of vaccines or diagnostic kits for preventing and treating acute lymphoblastic leukemia, lung cancer, liver cancer and the like.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Application of substance for reducing USP1 expression in preparation of medicine for treating children T-series acute lymphoblastic leukemia
ActiveCN111926018ASlow down proliferationPrevent proliferationOrganic active ingredientsAntineoplastic agentsLymphocyteOncology
The invention discloses application of a substance for reducing USP1 expression in preparation of a medicine for treating children T-series acute lymphocytic leukemia. The invention discloses short hairpin RNA (Ribonucleic Acid) for forming a stem-loop structure. The short hairpin RNA is shRNA-1 or shRNA-2. The deubiquitination enzyme USP1 has the effects of inhibiting cell apoptosis and promotingcell proliferation in T-series acute lymphocytic leukemia cells, and the invention proves that inhibition of USP1 expression can promote apoptosis of tumor leukemia cells and inhibit proliferation ofleukemia cells. The invention provides a novel way for the treatment of T-series acute lymphocytic leukemia, and the deubiquitinase USP1 is expected to become a potential target for anti-leukemia treatment and has a very wide application prospect in the medical field.
Owner:BEIJING CHILDRENS HOSPITAL AFFILIATED TO CAPITAL MEDICAL UNIV
Primers and kit for detecting specific gene HB-1 of acute B lymphocyte leukemia
InactiveCN103937901AImprove binding specificityLow GC contentMicrobiological testing/measurementDNA/RNA fragmentationLymphocyteB lymphoblastic leukemia
The invention relates to the field of diagnosis kits, and particularly relates to a pair of primer and kit for detecting the specific gene HB-1 of acute B lymphocyte leukemia. The primers for detecting the HB-1 and a probe are an upstream primer sequence as shown in SEQ ID NO:1, a downstream primer sequence as shown in SEQ ID NO:2 and a probe as shown in SEQ ID NO:3; the 5'end of a nucleotide sequence as shown in the SEQ ID NO:3 has a Fam mark, and the 3'end of the nucleotide sequence has a Tamra mark. The kit disclosed by the invention optimizes the probe sequence, simplifies the experimental step, realizes the single step of a detection method, enhances the experimental stability and realizes the quantitative detection of the gene HB-1.
Owner:PEKING UNIV FIRST HOSPITAL
Detection kit for relapse and drug resistance gene mutation of acute lymphoblastic leukemia (ALL) and application method thereof
ActiveCN108977541AGene selection targetsImprove applicabilityMicrobiological testing/measurementDiseaseUse medication
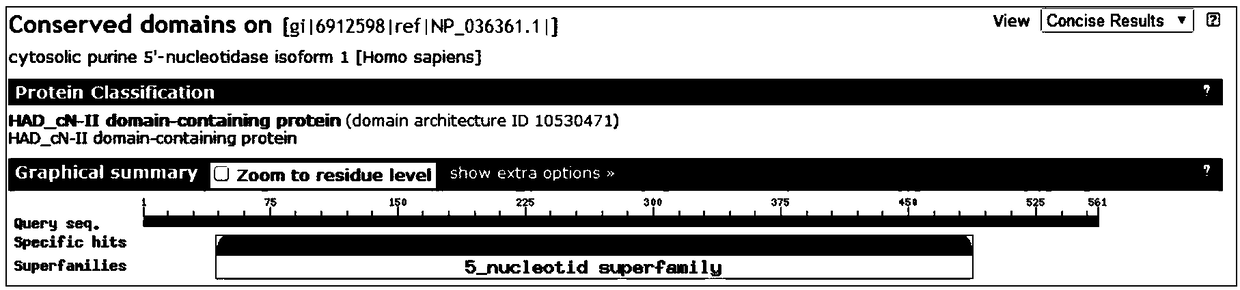
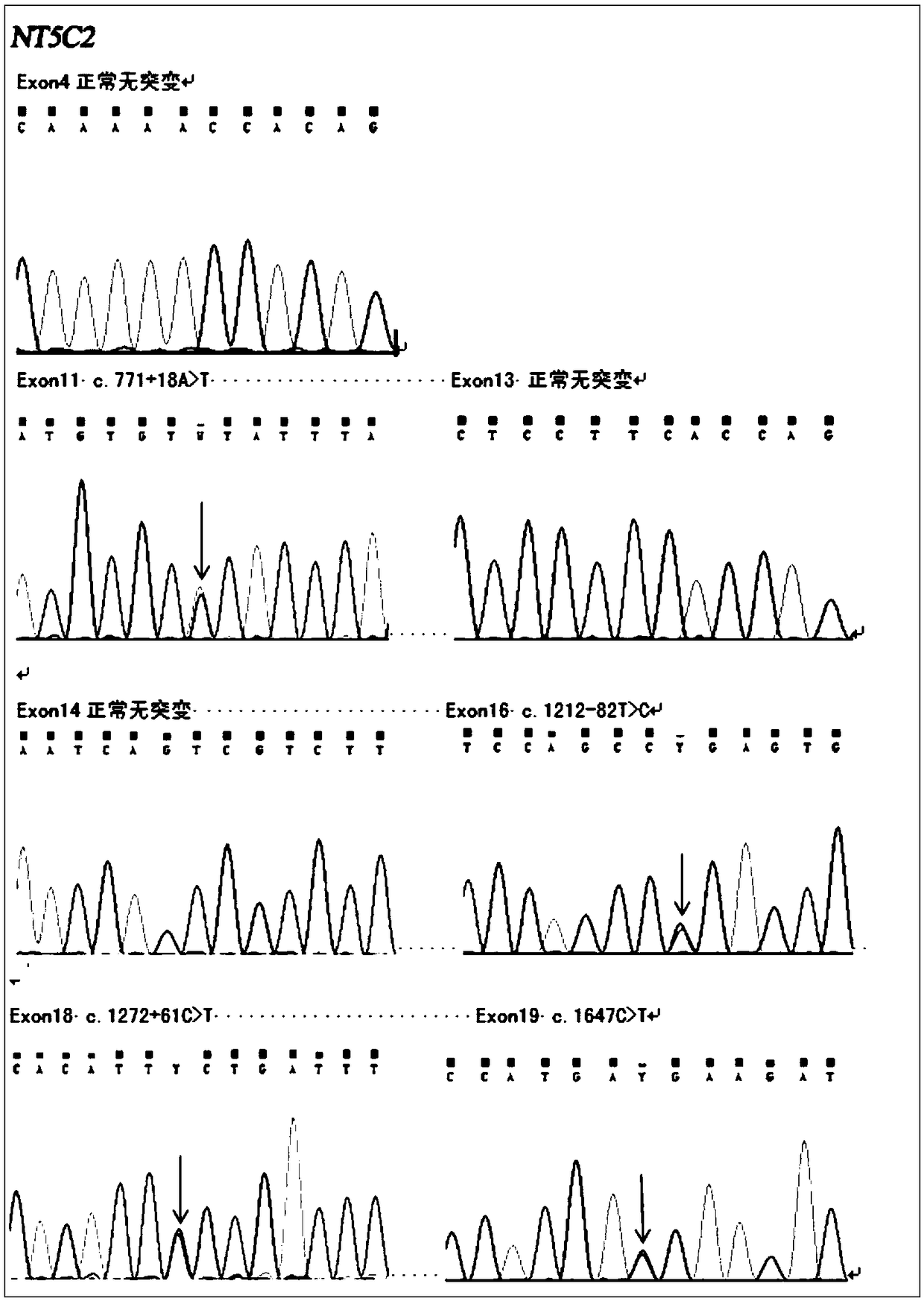
The invention belongs to the field of medicine and molecular detection, in particular to a detection kit for relapse and drug resistance gene mutation of acute lymphoblastic leukemia (ALL) and an application method thereof. The detection kit includes a specific primer for an NT5C2 gene mutation site, which can comprehensively and accurately detect the high mutation site of an NT5C2 gene. The detection result has strong guiding significance for clinical practice, can be used for rapid and accurate detection of relapse and drug resistance of ALL to avoid unnecessary therapeutic drugs and is of great importance in the filed of detection and treatment of ALL. Through mutation detection of the NT5C2 gene related to ALL relapse and drug resistance, the detection kit can better guide the medication and treatment of clinicians, and can be used as an auxiliary means for diagnosis of relapse and drug resistance of the disease.
Owner:北京海思特医学检验实验室有限公司
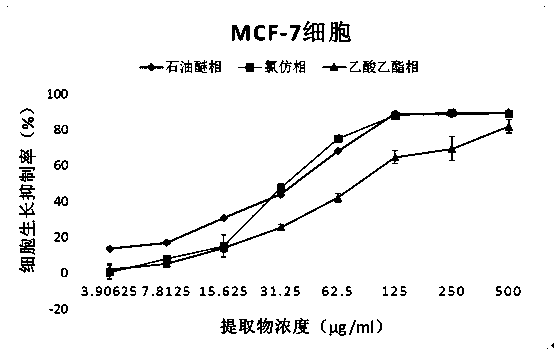
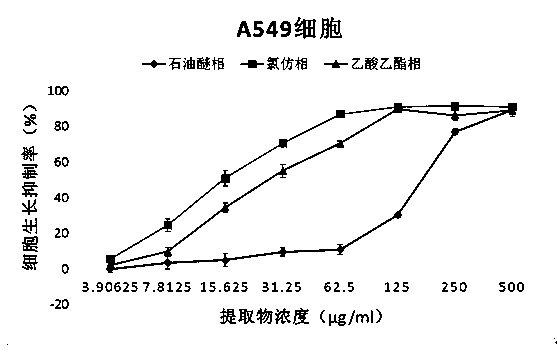
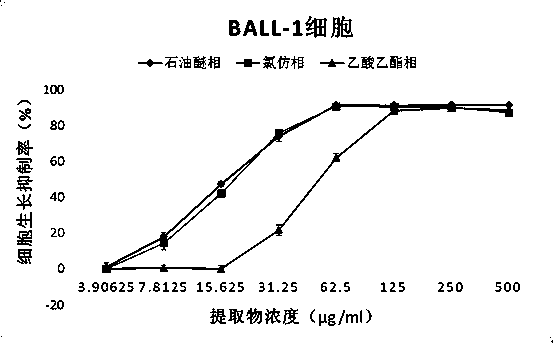
Application of fatsia japonica branch and leaf extractives to anti-cancer drug preparation and preparation of fatsia japonica branch and leaf extractives
InactiveCN104257708ASimple methodLow costAntineoplastic agentsPlant ingredientsIllicium verumEthyl acetate
The invention discloses application of fatsia japonica branch and leaf extractives to anti-cancer drug preparation and preparation of the fatsia japonica branch and leaf extractives, and belongs to the technical field of medicines and natural medicines. A preparation method of the fatsia japonica branch and leaf extractives includes the following steps: ethyl alcohol with 95-percent concentration serves as extracting agents; an ultrasonic extraction method is adopted for extracting fatsia japonica branches and leaves; total ethyl alcohol extractives of the fatsia japonica branch and leaf extractives are obtained; petroleum ether, chloroform and ethyl acetate are sequentially used for extracting the total ethyl alcohol extractives; a petroleum ether part, a chloroform part, an ethyl acetate part and an extraction remaining part are obtained. Meanwhile, supersonic extraction is carried out on the fatsia japonica branches and leaves obtained after extraction with the ethyl alcohol is carried out with distilled water, and an aqueous extract part is obtained. The five extraction parts of the fatsia japonica branches and leaves are of great significance in preparing medicines for treating the breast cancer, the non-small cell lung cancer and the human B-cell acute lymphoblastic leukemia.
Owner:KUNMING UNIV OF SCI & TECH
Use of succinyl hydrazine compounds and compositions thereof in preparation of drug for treating leukemia
InactiveCN1990462AExplore effective waysVerify biological activityOrganic chemistryAmide active ingredientsHydrazine compoundChronic granulocytic leukemia
The invention relates to succinyl hydrazine compounds derivative and medical compound containing them; and the application of it in preparing medicine that resists leukemia. The succinyl hydrazine compounds derivative shows inhibiting action to BCR-Ab1 tyrosine kinase activity through pharmacological test. It is characterized by high effecivity and low toxicity, and can be widly used to prepare medicine that treats chronic granulocytic leukemia (CML) and philadelphia chromosome positive acute lymphoblastic leukemia, especially medicine treating patient that generates drug resistance to Gleevec.
Owner:INST OF HEMATOLOGY & BLOOD HOSPITAL CHINESE ACAD OF MEDICAL SCI
ALL prognostic related gene mutation detection method, primers and kit
InactiveCN106148553AThe detection process is fast and sensitiveEasy to operateMicrobiological testing/measurementDNA/RNA fragmentationBlood collectionMutation detection
The embodiment of the invention provides an ALL prognostic related gene mutation detection method, primers and a kit. PCR and sequencing technologies are used, specific primers are mainly used for amplifying specific exon sections of CREBBP genes in genome DNA, sequencing is conducted on target fragments obtained through amplification through a sequencing reaction, and whether mutation exists or not is detected by comparing the obtained sequence with a standard genetic sequence. The detection process is rapid and sensitive, operation is easy, the accuracy is high, and the detection result is definite and clear; the detection specificity is high, a gene mutation detection method which is the most accurate at present is adopted, and purposiveness is high; the blood collection amount of a detection specimen is low, a detected object himself / herself is benefited, fewer experimental instruments are used in the detection process, and the cost is reduced. The invention aims at obtaining analytical data capable of being used for medication guidance and prognosis evaluation of acute lymphoblastic leukemia, and the analytical data can play an important role in the field of medical detection.
Owner:北京海思特医学检验实验室有限公司
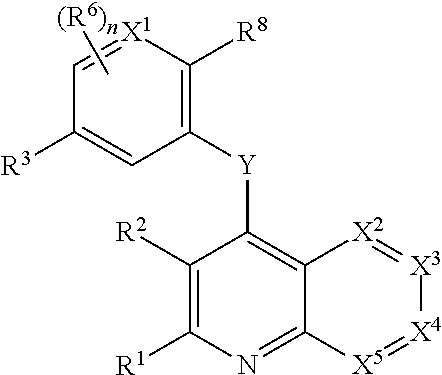
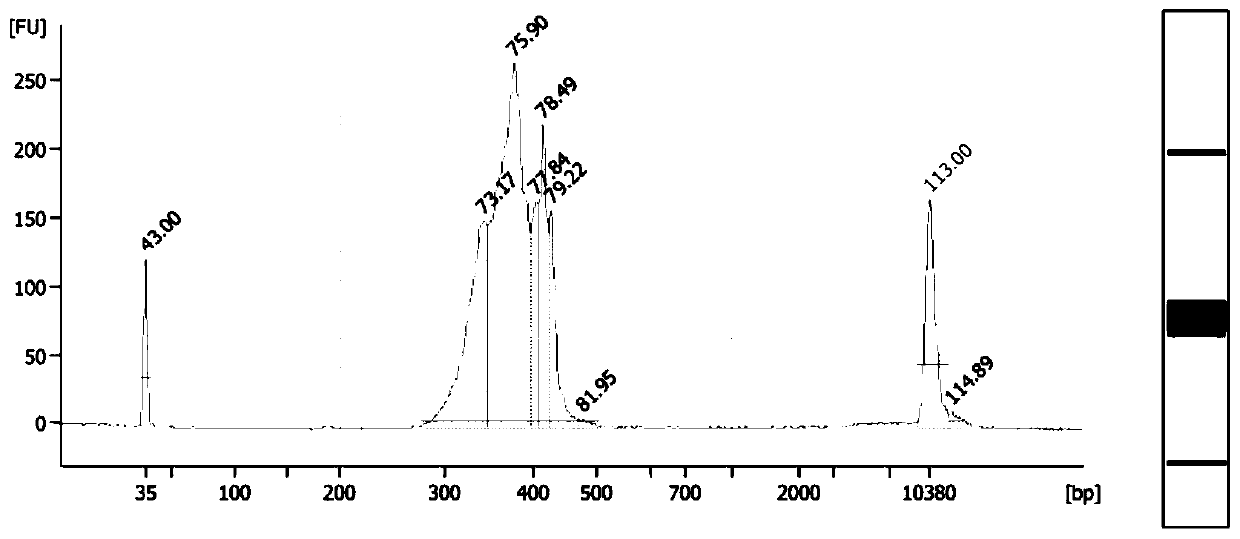
New crystal forms of 4-[6-methoxy-7-(3-piperidin-1-yl-propoxy)quinazolin-4-yl]piperazine-1-carboxylic acid(4-isopropoxyphenyl)-amide
4-[6-Methoxy-7-(3-piperidin-1-yl-propoxy)quinazolin-4-yl]piperazine-1-carboxylic acid (4-isopropoxy A crystalline form of phenyl)-amide sulfate, which can be used for pharmaceutical applications. The particular single crystal form of the sulfate salt was defined by various properties and physical tests. Also disclosed are methods for preparing said sulfates, and the use of these salts to inhibit the activity of excess tyrosine kinases in the host for the treatment of many diseases, including cardiovascular diseases (e.g., arteriosclerosis and vascular infarction) ), tumors (eg, leukemias such as acute lymphoblastic leukemia), glomerulosclerotic fibrotic diseases and inflammation, and conventional treatment of cell expansion-type diseases.
Owner:MILLENNIUM PHARMA INC
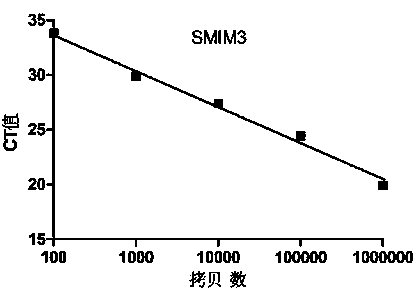
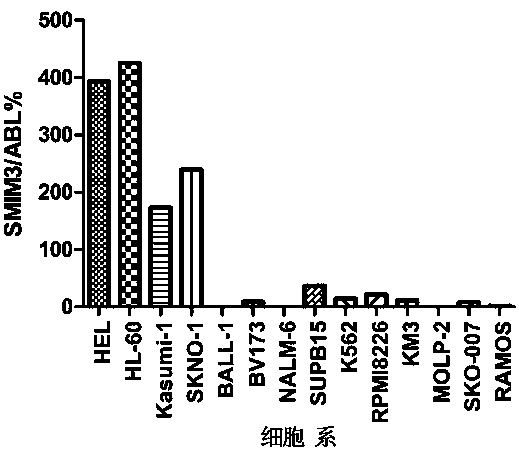
Primer probe set, kit and method for quantitative detection of expression level of small integral membrane protein 3 (SMIM3) gene
PendingCN111100931AHigh sensitivityImprove reliabilityMicrobiological testing/measurementDNA/RNA fragmentationForward primerIntegral membrane protein
The invention discloses a primer probe set, kit and method for quantitative detection of an expression level of a small integral membrane protein 3 (SMIM3) gene. The primer probe set comprises a component A and a component B; the component A includes a forward primer SMIM3-FP, a reverse primer SMIM3-RP and a probe SMIM3-probe; and the component B includes a forward primer ABL1-FP, a reverse primerABL1-RP and a probe ABL1-probe. The primer probe set, the kit and the method have the advantages that quantitative detection of the relative expression level of the SMIM3 gene in a cell is realized;moreover, the sensitivity of the SMIM3 gene and the sensitivity of an ABL1 gene both reach up to 100 copies; the sensitivity is high, results are accurate, and the reliability is high; a new auxiliarydiagnosis method or auxiliary identification method is provided for hematologic tumor cells (especially acute myelogenous leukemia and acute lymphoblastic leukemia); and the primer probe set, the kitand the method have broader application prospects.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
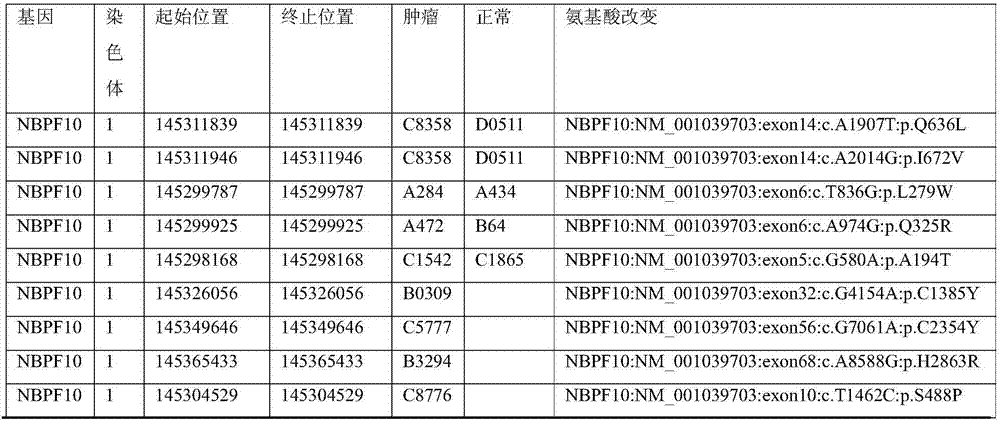
Kit for simultaneously detecting mutations of genes NBPF10, TSFM, PRB2 and DIAPH1
ActiveCN107974502AMicrobiological testing/measurementDNA/RNA fragmentationGermline mutationMRD Negative
The present invention discloses a kit for simultaneously detecting mutations of genes NBPF10, TSFM, PRB2 and DIAPH1, wherein the kit comprises a plurality of multi-gene capture probes, can be used fordetecting the somatic mutations and the germline mutations at the tumor genome level, can be used for the assisted diagnosis of patients with hematological tumors (especially acute lymphoblastic leukemia patients), the monitoring of minimal residual diseases, the evaluation of prognosis, and the like, and can provide important effect in the field of medical detection, wherein the detection methodis simple and sensitive.
Owner:PEOPLES HOSPITAL PEKING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
































![New crystal forms of 4-[6-methoxy-7-(3-piperidin-1-yl-propoxy)quinazolin-4-yl]piperazine-1-carboxylic acid(4-isopropoxyphenyl)-amide New crystal forms of 4-[6-methoxy-7-(3-piperidin-1-yl-propoxy)quinazolin-4-yl]piperazine-1-carboxylic acid(4-isopropoxyphenyl)-amide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/28c31858-7cfb-4830-a1d2-ac46aaf78a1a/s2006800232271e00011.PNG)
![New crystal forms of 4-[6-methoxy-7-(3-piperidin-1-yl-propoxy)quinazolin-4-yl]piperazine-1-carboxylic acid(4-isopropoxyphenyl)-amide New crystal forms of 4-[6-methoxy-7-(3-piperidin-1-yl-propoxy)quinazolin-4-yl]piperazine-1-carboxylic acid(4-isopropoxyphenyl)-amide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/28c31858-7cfb-4830-a1d2-ac46aaf78a1a/s2006800232271e00021.PNG)
![New crystal forms of 4-[6-methoxy-7-(3-piperidin-1-yl-propoxy)quinazolin-4-yl]piperazine-1-carboxylic acid(4-isopropoxyphenyl)-amide New crystal forms of 4-[6-methoxy-7-(3-piperidin-1-yl-propoxy)quinazolin-4-yl]piperazine-1-carboxylic acid(4-isopropoxyphenyl)-amide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/28c31858-7cfb-4830-a1d2-ac46aaf78a1a/s2006800232271e00031.PNG)