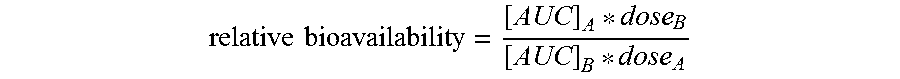Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Danazol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used in women to treat pelvic pain and infertility due to a certain uterus disorder (endometriosis) and also to treat breast pain/tenderness/nodules due to a certain breast condition (fibrocystic breast disease). It is also used in both men and women to prevent swelling of the abdomen/arms/legs/face/airway due to a certain congenital disease (hereditary angioedema).
Pharmaceutical Compositions Comprising Danazol
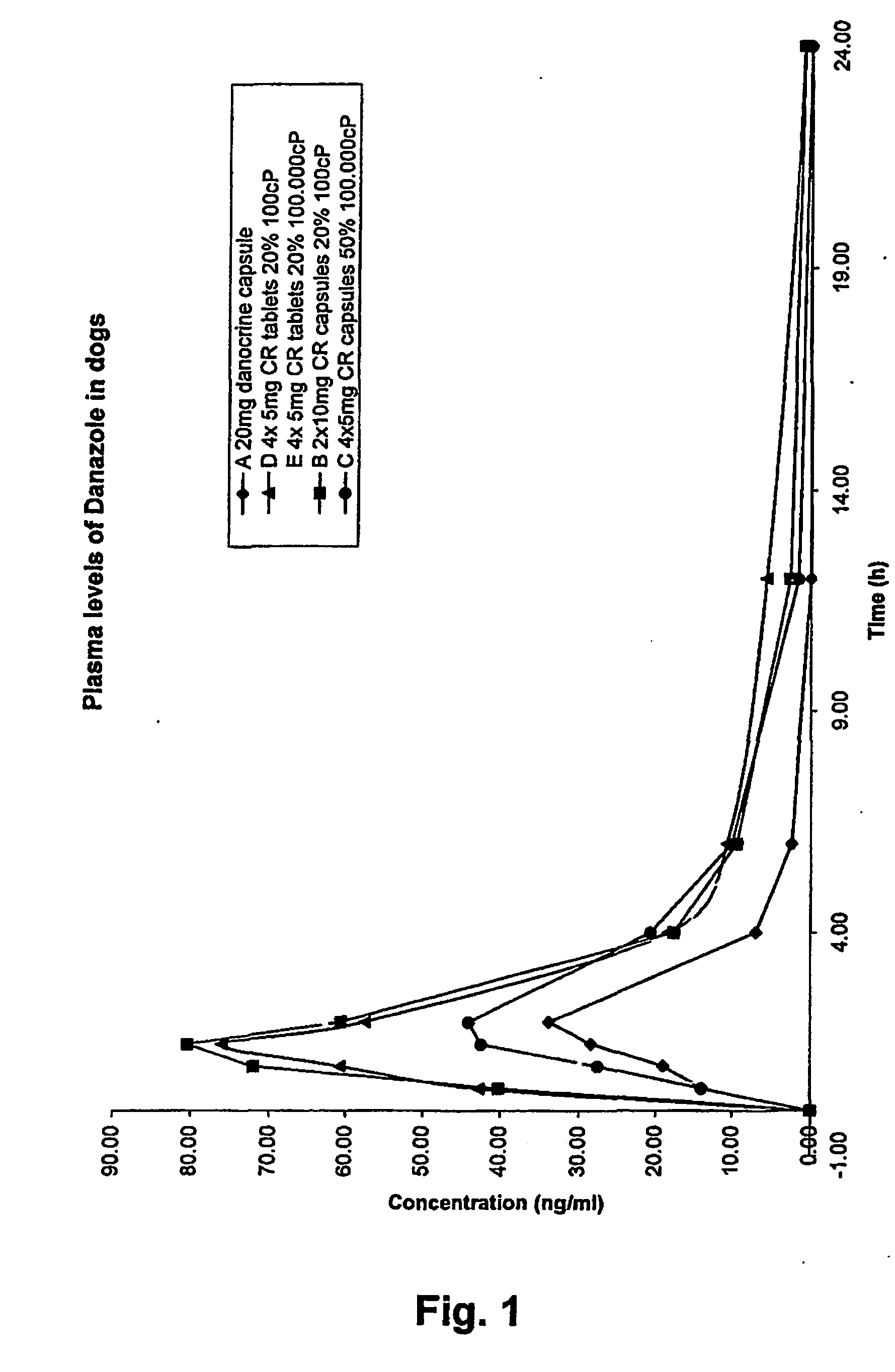
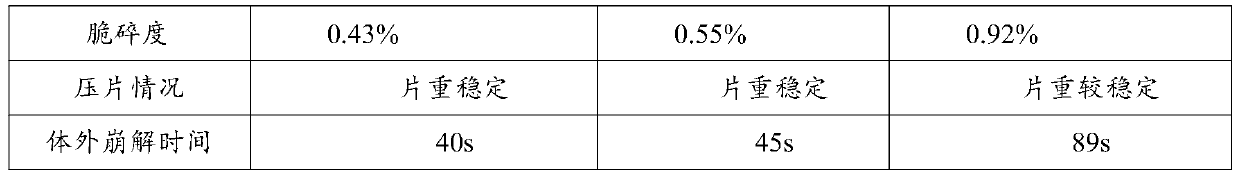
A controlled release pharmaceutical comprising danazol has the property of slow release of danazol over an extended period of time and markedly increased bioavailability compared to commercially available danazol-containing products. The pharmaceutical composition comprises danazol dissolved in a solid vehicle or carrier and is especially suitable for oral solid dosage forms. The composition significantly reduces food effect and may reduce side effects.
Owner:LIFECYCLE PHARMA AS
Danazol for treatment of hypogonadism in the adult male
InactiveUS20050143362A1Enhancing testosterone activityReducing and treating hypogonadismOrganic active ingredientsCapsule deliveryPhysiologyAndrogen
The method of use of danazol for enhancing the testosterone activity in the adult male. In particular, androgen activity levels are increased in the adult male suffering from hypogonadism by pharmaceutical compositions of danazol that are disclosed.
Owner:MCLANE MICHAEL W
Preparation method of danazol
The invention discloses preparation methods of danazol and an intermediate thereof. The preparation method of danazol is prepared by the steps of taking androstenedione as a starting raw material, and carrying out 3-site enol etherification, 17-site carbonyl ethinylation, 3-site hydrolysis, 2-site methylidynel hydroxylation and oximation to obtain danazol. The 3-site enol etherification comprises firstly carrying out a reaction of androstenedione and triethyl orthoformate for 4-10 h in the presence of absolute ethyl alcohol and p-toluenesulfonic acid and at the temperature of 30-50 DEG C, then adding triethylamine at the temperature of 0-10 DEG C, and continuing to carry out a reaction for 0.2-1 h; the 17-site carbonyl ethinylation comprises firstly carrying out a reaction of a potassium hydroxide powder for 1-2 h in an acetylene airflow and at the temperature of 5-10 DEG C, and then carrying out a reaction with the 3-site enol etherified product for 2-4 h in the presence of tetrahydrofuran and a catalyst, at the temperature of 15-30 DEG C and in the acetylene airflow. The 3-site enol etherification is mild in reaction conditions and relatively high in yield, and the 17-site carbonyl ethynylation is relatively high in reaction yield and relatively short in time.
Owner:佳尔科生物科技南通有限公司
Composition of danazol semisolid skeleton preparation
The present invention discloses a composition of danazol semi-solid skeleton preparation, its composition includes danazol 0.5-10 wt%, surfactant whose HLB is greater than above 12 1-98.5 wt% and carrier of semi-solid skeleton 1-98.5%. Said composition is good is dissolution property, can be used for curing endometriosis, and can have obvious therapeutic effect, also can be used for fibroadenosis, spontaneous thrombopenic purpura, heteditary angioneuotic edema and systemic lupus erythematosus. Said invention possesses the features of low dosage, quick absorption, high blood concentration and biological utilization.
Owner:SHANGHAI INST OF PHARMA IND
Method of preparing ultra-fine danazol powder
InactiveCN101292958AFine granularityUniform compositionPowder deliveryOrganic active ingredientsUltra fineDesolvation
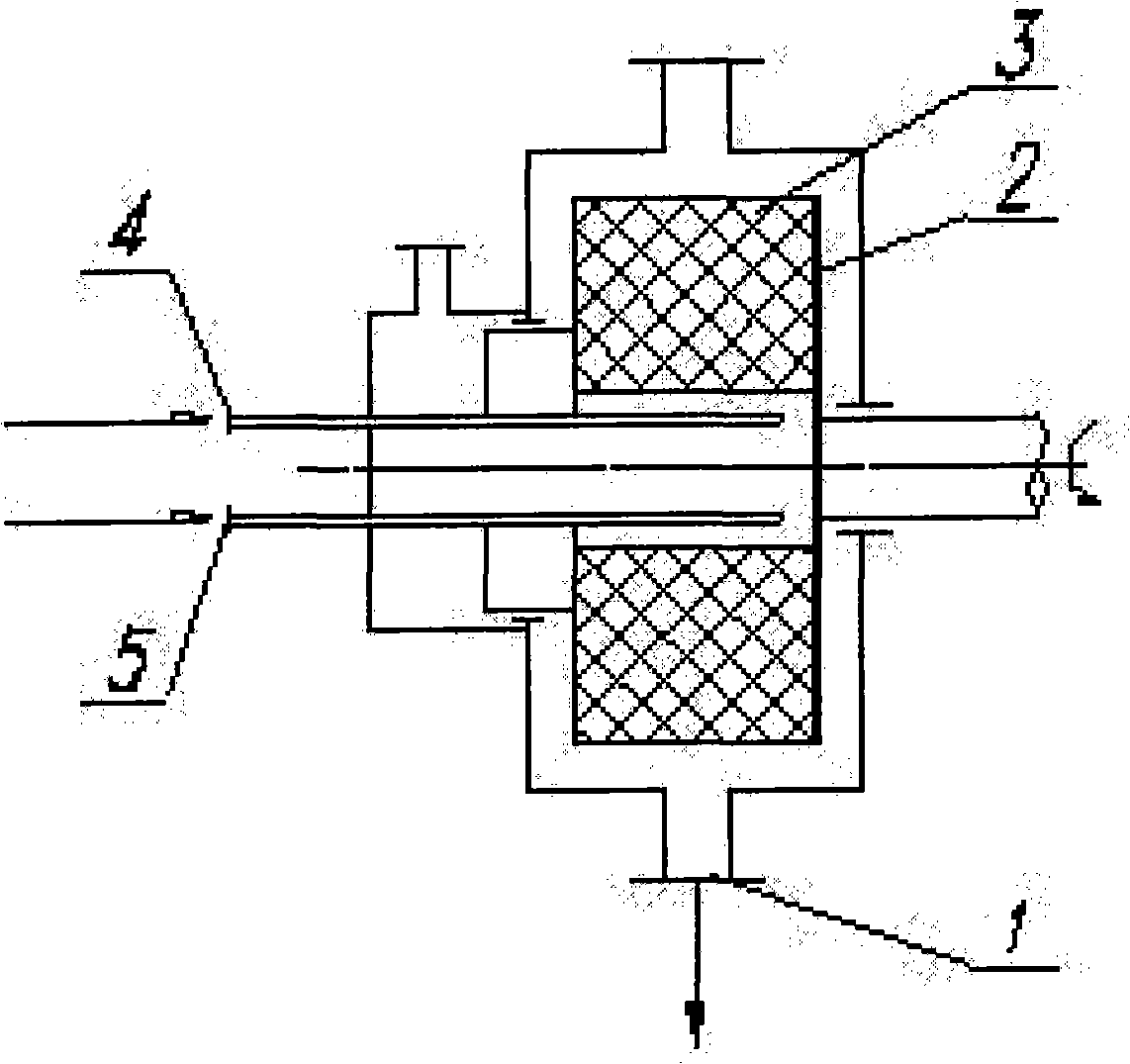
A preparation method of ultra-fine danazol powder belongs to the method of drug micronization. The method is that danazol solution and desolvation are first put into a supergravity rotating bed through a solution entrance 4 and a desolvation entrance 5 respectively at the same time, and the recrystallized danazol paste by the desolvation flows out from a discharge hole 1; the danazol paste is collected, filtered and dried to obtain the ultra-fine danazol powder. The method can obtain the ultra-fine danazol powder of controlled average grain size, controlled shape and narrow grain size distribution.
Owner:BEIJING UNIV OF CHEM TECH +1
Vaginal danazol combined with non steroidal anti inflammatory drugs (nsaids) compositions
InactiveUS20150283066A1Improve adhesionIncrease release rateBiocidePeptide/protein ingredientsImmediate releaseBULK ACTIVE INGREDIENT
The present invention discloses a pharmaceutical composition useful for treating a gynecological condition comprising (a) an active ingredient selected from a group consisting of at least one non-steroidal anti-inflammatory drug (NSAID), danazol and a combination thereof, (b) at least one pharmaceutically acceptable carrier or excipient. In a core aspect of the invention that the composition is adapted to be vaginally administrable further wherein said composition is formed in an immediate release form. Kits and methods using the aforementioned composition are also disclosed.
Owner:MEDIGLOBE
Medicine for treating endometriosis and preparation method thereof
InactiveCN101190265ASuitable objectsFormula features are obviousInanimate material medical ingredientsPill deliveryClinical efficacyPelvic pain
The invention relates to a drug used for treating endometriosis. The drug is prepared by the following raw materials by weight: 2.80-3.20 parts of pieplant, 2.80-3.20 parts of turtle shell, and 1.30-1.70 parts of amber. The invention discloses a method of producing the drug used for treating endometriosis. The drug has the functions of dissipating blood stasis and purging the bowels, is mainly used for treating endometriosis the indications of which are dysmenorrhea, pelvic pain, straining feeling in anus, sore waist and constipation, dark tongue or with ecchymosis, thin tongue coating, thready pulse or pulse string small. Compared with the western medicine danazol, the invention has similar clinical curative effects but with fewer adverse effects and can be easily accepted by patients.
Owner:北京羚锐伟业科技有限公司 +1
Danazol vaginal tablet process and quality control method
The invention relates to a preparation method and a quality control method of a new drug, Danazol Vaginal Tablets, for treating endometriosis with clear dysmenorrhea symptom but light physical sign. The drug can be delivered via cavitary mucosa, so as to prevent or relieve the possible adverse reactions due to systemic drug delivery; the effective drug concentration at lesion site is high, so as to be in favor of exert curative action; and the added lactic acid-calcium lactate buffer system results in the drug with pH of 3.5-4.0 that is similar to the physiological pH in vagina, so as to effectively retain physiological environment in vagina, provide existing conditions suitable for vaginal beneficial bacteria, and promote rehabilitation.
Owner:王世锋
Medicinal chitin fiber for preventing mammary gland hyperplasia, and its preparation and use
ActiveCN1936128ADoes not affect external beautyIn line with human needsFilament/thread formingBrassieresFiberIsoflavones
The invention discloses a method to make medicine using Chitin-fiber used for prevent hyperplasia of mammary glands and the application. It contains Chitin, medicine and medicine adsorbing accelerant. The medicine includes one or more from Tamoxifen, isoflavone, bromocriptine and Danazol. The medicine adsorbing accelerant is one or the compound from azone, oleic acid, N-methyl-2- pyrrolidone, propylene glycol, and borneol. The Chitin contains weight proportion of A, medicine contains weight proportion of B and medicine adsorbing accelerant contains weight proportion of C, and 60 D01F 9 / 00 D01F 1 / 10 D01D 5 / 00 A61L 15 / 28 A41C 3 / 00 A41B 9 / 12 0 6 1 2006 / 8 / 17 1936128 2007 / 3 / 28 100447320 2008 / 12 / 31 2008 / 12 / 31 2008 / 12 / 31 Lifangjian Medical Sci. & Tech. Consulting (Shanghai) Co., Ltd. Shanghai 201108 Zhu Limin Fan Zaixia dongmei 31208
Owner:ZHEJIANG LANGSHA UNDERWEAR
Danazol tablet composition
InactiveCN108785264ASmooth appearanceHigh dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsCarboxymethyl starchSulfate
The invention relates to a danazol tablet composition, and belongs to the technical field of medicine preparations. The danazol tablet composition in a unit dose comprises 100 mg of danazol, 1.4-2.6 mg of lecithin, 1.2-1.8 mg of sodium lauryl sulfate, 36-64 mg of lactose, 8-15 mg of low-substituted hydroxypropyl methyl celluloses, 4-8 mg of sodium carboxymethyl starch and 1.0-1.8 mg of magnesium stearate. The danazol tablet composition has the advantage that the clinical requirements can be met by the danazol tablet composition.
Owner:WEIHAI GUANBIAO INFORMATION TECH
Preparation method of danazol
The invention provides a preparation method of danazol. According to the preparation method of the danazol, androstenedione is used as an initial raw material, through 2-position secondary hydroxymethylation reaction, 17-position carbonyl ethinylation reaction and oximation reaction, the danazol can be prepared, and the 2-position secondary hydroxymethylation reaction and the 17-position carbonylethinylation reaction are completed in one step. The preparation method of the danazol has the advantages of being safe, short in reaction step, high in yield, efficient in reaction and the like, andin the reaction, no flammable and combustible acetylene gas with huge potential safety hazards is used, so that preparation in which potassiumacetylide is replaced by ethynylmagnesium bromide is safer. Meanwhile, the reaction which needs to be completed in four steps before is completed in one step, the reaction is more efficient, meanwhile, operation is simple, and reaction time is short.
Owner:JIANGSU LIANHUAN PHARMA
Methods of treatment of diseases
InactiveUS9351979B2Increasing and prolonging formationOrganic active ingredientsSenses disorderDiseaseCytoskeleton
Owner:AMPIO PHARMA
Endometriosis treatment
InactiveUS20130184245A1Organic active ingredientsOintment deliveryIntravaginal administrationDanazol
A pharmaceutical composition comprises at least one nonlipoidal internal phase and at least one lipoidal external phase that is bioadhesive to a vaginal mucosal surface, and comprises danazol in an amount of about 3% to about 30% by weight of the composition, wherein upon application of the composition to the vaginal mucosal surface the danazol is released over a period of about 1 to about 10 days. The composition is useful for intravaginal administration to treat a condition such as endometriosis for which danazol is indicated.
Owner:LUMARA HEALTH IP
Purification method of small-grain size danazol
The invention relates to a purification method of small-grain size danazol, and belongs to the technical field of crude drug preparation. According to the technical scheme, firstly, crude danazol is added into acetone, and stirring is conducted for dissolution; activated carbon which is 1-3% of the crude danazol is added, and stirring and filtering are conducted; filter liquor is cooled to -10-0 DEG C, the temperature is maintained, and ethanol water which is 1.2-3 times of the solvent in the step 1 is added in a fed-batch manner; stirring is conducted for crystallization; filtering is conducted, and a filter cake is washed with the ethanol water in the step 2; drying is conducted at 80 DEG C. By means of the method, a high-purity danazol crude drug with a small grain size is obtained.
Owner:WEIHAI GUANBIAO INFORMATION TECH
Use of kakonein in preparing medicine for treating endometriosis
The present invention discloses the application of puerasin in preparing medicine for treating endometriosis. Animal test proves the obvious endometriosis treating effect of puerasin, and what is more important is that puerasin possesses inapproachable safety compared with danazol. With high treating effect and basically no adverse effect, puerasin possesses excellent application foreground in treating endometriosis.
Owner:俞超芹 +1
Topical Administration of Danazol
InactiveUS20100152146A1Reducing abundance and appearanceReduce appearanceCosmetic preparationsOrganic active ingredientsLotionPharmaceutical formulation
Pharmaceutical preparations for topical or local administration of drugs directly to the skin for treatment of disorders of the subcutaneous fatty tissue, in particular in cases of cellulite, are disclosed herein. In a preferred embodiment, the drug is danazol or gastrinone. In another embodiment, the drug is danazol in combination with an aromatase inhibitor or an estrogen compound. The preferred formulations contain drugs in the form of micro or nanoparticles, which may be formed of drug alone or in combination with an excipient or carrier. The excipient or carrier may modify the release rates or enhance absorption into the affected area. The drug formulation may be in the form of a cream, lotion, ointment, gel or emulsion, solution or foam.
Owner:FEMMEPHARMA HLDG CO INC
Intra-uterus administered medicinal stick of danazol and preparation process thereof
InactiveCN1965842AStable release rateOrganic active ingredientsPharmaceutical delivery mechanismDiseaseMedicine
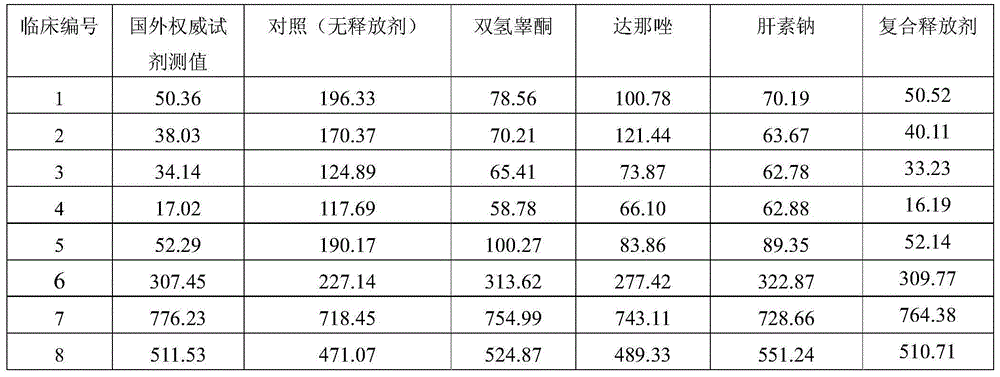
The invention relates to a Danaco drug rod for feeding agent in uterus, wherein it uses silicon gel as carrier, uses polychloroethylene rod as support, and it is formed by 20.0% Danaco, 40.0% silicon gel, and 40.0% silicon gel vulcanizer. The production comprises that: mixing drug and silicon gel uniformly to be coated on support to prepare rod agent; the rod agent can release drug at 1-7% of total amount each day, to function for three months. The invention can express local drug function, to function the uterus directly. And it uses slow-release agent to release drug stably.
Owner:ZHEJIANG UNIV
Use of wasp's nest in preparing medicine for preparing endometriosis
The present invention discloses the application of wasp's nest in preparing medicine for treating endometriosis. Animal test proves the obvious endometriosis treating effect of wasp's nest, and what is more important is that wasp's nest possesses inapproachable safety compared with danazol. With high treating effect and basically no adverse effect, wasp's nest possesses excellent application foreground in treating endometriosis.
Owner:SHANGHAI CHANGHAI HOSPITAL
Endometriosis treatment
InactiveUS20080287408A1Effective treatmentOrganic active ingredientsOintment deliveryIntravaginal administrationDanazol
A pharmaceutical composition comprises at least one nonlipoidal internal phase and at least one lipoidal external phase that is bioadhesive to a vaginal mucosal surface, and comprises danazol in an amount of about 3% to about 30% by weight of the composition, wherein upon application of the composition to the vaginal mucosal surface the danazol is released over a period of about 1 to about 10 days. The composition is useful for intravaginal administration to treat a condition such as endometriosis for which danazol is indicated.
Owner:DRAGTEK CORP
A kind of preparation method of danazol
ActiveCN109517027BSafe preparationEfficient preparationSteroidsProcess engineeringCombinatorial chemistry
The invention provides a preparation method of danazol. According to the preparation method of the danazol, androstenedione is used as an initial raw material, through 2-position secondary hydroxymethylation reaction, 17-position carbonyl ethinylation reaction and oximation reaction, the danazol can be prepared, and the 2-position secondary hydroxymethylation reaction and the 17-position carbonylethinylation reaction are completed in one step. The preparation method of the danazol has the advantages of being safe, short in reaction step, high in yield, efficient in reaction and the like, andin the reaction, no flammable and combustible acetylene gas with huge potential safety hazards is used, so that preparation in which potassiumacetylide is replaced by ethynylmagnesium bromide is safer. Meanwhile, the reaction which needs to be completed in four steps before is completed in one step, the reaction is more efficient, meanwhile, operation is simple, and reaction time is short.
Owner:JIANGSU LIANHUAN PHARMA
Medical protein fiber for preventing and treating proliferation of mammal gland and its preparation method and application
ActiveCN100447319CRich sourcesLow costOrganic active ingredientsConjugated cellulose/protein artificial filamentsTextile fiberIsoflavones
This invention involves one kind of medicinal purposes protein textile fiber which is used for preventing and curing hyperplasia of mammary glands, and the process and application. The compound according to textile fiber gross is as follows: percentage A is protein, percentage B is high polymer, percentage C is medicine, percentage D is medicine that passes the skin absorption promoter, and the percentage is: 1<=A<=80,1<=B<=80,0<C <=20,0<D<=10. Among them, the high polymer is a kind of or a combination of polyvinyl alcohol, polyacrylonitrile, cellulose, polyvinyl acetate; the medicine can be one or a combination of xifen, isoflavone, bromocriptine and danazol; the medicine skin penetration enhancer can also be one or a combination of nitrogen alkone, oleic acid, N- methyl - 2- pyrrolidone, trimethylene glycol, or borneol.
Owner:义乌立芙纺织有限公司
Medicinal chitin fiber for preventing mammary gland hyperplasia, and its preparation and use
ActiveCN100447320CDoes not affect external beautyIn line with human needsBrassieresFilament/thread formingFiberIsoflavones
The invention discloses a method to make medicine using Chitin-fiber used for prevent hyperplasia of mammary glands and the application. It contains Chitin, medicine and medicine adsorbing accelerant. The medicine includes one or more from Tamoxifen, isoflavone, bromocriptine and Danazol. The medicine adsorbing accelerant is one or the compound from azone, oleic acid, N-methyl-2- pyrrolidone, propylene glycol, and borneol. The Chitin contains weight proportion of A, medicine contains weight proportion of B and medicine adsorbing accelerant contains weight proportion of C, and 60<A<=99, 0<B<=30, 0<C<=10. The invention also discloses the manufacturing method for the fiber and the method to make bra from the fiber.
Owner:ZHEJIANG LANGSHA UNDERWEAR
Pharmaceutical composition containing danazol and application thereof
ActiveCN111588699AEasy to swallowImprove medication complianceOrganic active ingredientsPill deliveryFormularyUse medication
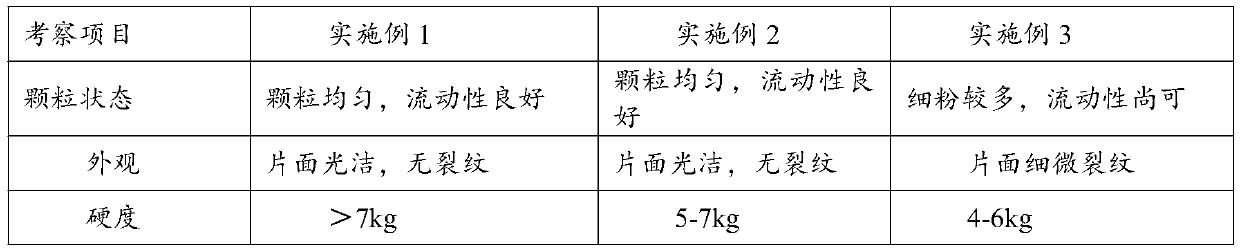
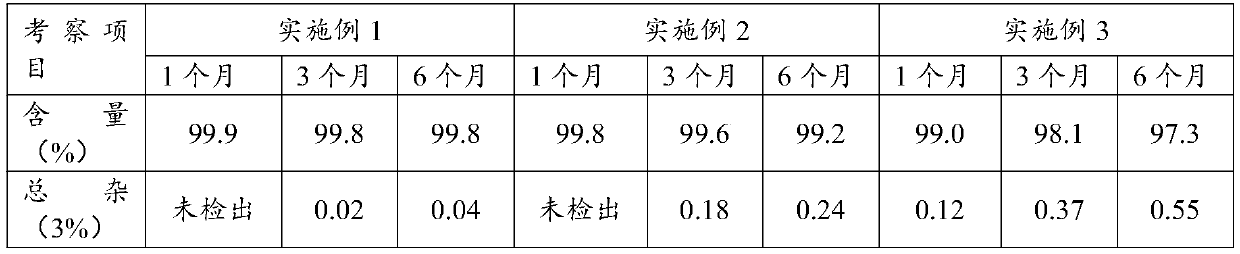
The invention relates to a solid pharmaceutical composition containing danazol. The solid pharmaceutical composition comprises the following components in percentage by weight: 70-99% of danazol and 1-10% of a pharmaceutically acceptable carrier. The pharmaceutically acceptable carrier is optionally selected from any one or a combination of a diluent, a lubricant, an adhesive, a disintegrating agent and a glidant. By scientifically screening and optimizing the formula of the solid pharmaceutical composition, firstly, the content of danazol is increased, a clinical medication scheme is simplified, and the medication compliance of a patient is remarkably improved; 2, the hardness and friability of the tablet are obviously improved, and the product quality is improved; 3, potential problems of impurity limit control and the like possibly caused by auxiliary materials are remarkably reduced, and the use safety of the medicine is improved; and 4, the dissolution rate of the drug is improved, so that the danazol can be absorbed by a human body to the maximum extent.
Owner:HEILONGJIANG UNIV OF CHINESE MEDICINE
Method of preparing ultra-fine danazol powder
InactiveCN101292958BParticle size controllableShape is easy to controlPowder deliveryOrganic active ingredientsUltra fineDesolvation
Owner:BEIJING UNIV OF CHEM TECH +1
Use of wasp's nest in preparing medicine for preparing endometriosis
The present invention discloses the application of wasp's nest in preparing medicine for treating endometriosis. Animal test proves the obvious endometriosis treating effect of wasp's nest, and what is more important is that wasp's nest possesses inapproachable safety compared with danazol. With high treating effect and basically no adverse effect, wasp's nest possesses excellent application foreground in treating endometriosis.
Owner:SHANGHAI CHANGHAI HOSPITAL
A testosterone detection reagent based on microparticle chemiluminescence immunoassay technology
ActiveCN103954779BHigh detection sensitivityImprove detection efficiencyChemiluminescene/bioluminescenceBiological testingMicrosphereMicroparticle
Owner:DIRUI MEDICAL TECH CO LTD
The preparation method of danazol
The invention discloses a preparation method of danazol and its intermediate, which uses androstenedione as a starting material, undergoes etherification of 3-position enol, 17-position carbonyl acetylation, 3-position hydrolysis, 2-position Secondary hydroxylation and oximation to obtain danazol. The etherification of the 3-position enol is firstly reacted with androstenedione and triethyl orthoformate in the presence of absolute ethanol and p-toluenesulfonic acid at a temperature of 30-50°C for 4-10 hours; Add triethylamine at a temperature of 10°C to continue the reaction for 0.2~1h; the ethynylation of the 17-position carbonyl is to first react potassium hydroxide powder in an acetylene stream at a temperature of 5~10°C for 1~2h; and then react with the 3-position The enol etherification product is reacted in the acetylene gas stream at a temperature of 15-30° C. for 2-4 hours in the presence of tetrahydrofuran and a catalyst. The reaction conditions of the 3-position enol etherification reaction in the present invention are mild, and the yield is high, while the 17-position carbonyl ethynylation reaction has a high yield and a short time.
Owner:佳尔科生物科技南通有限公司
Use of kakonein in preparing medicine for treating endometriosis
The present invention discloses the application of puerasin in preparing medicine for treating endometriosis. Animal test proves the obvious endometriosis treating effect of puerasin, and what is more important is that puerasin possesses inapproachable safety compared with danazol. With high treating effect and basically no adverse effect, puerasin possesses excellent application foreground in treating endometriosis.
Owner:俞超芹 +1
Pharmaceutical polyacrylonitrile fiber for preventing and curing mammary gland hyperplasia, preparation and application thereof
ActiveCN100503909CRich sourcesLow costOrganic active ingredientsPharmaceutical delivery mechanismSide effectMethyl group
The invention relates to a medicinal polyacrylonitrile fiber for preventing hyperplasia of mammary glands, its manufacturing method and application. A medicinal polyacrylonitrile fiber for preventing hyperplasia of mammary glands, wherein the proportion of polyacrylonitrile to A in the total weight of the fiber is 60<A≤99, the proportion of medicine to B in the total weight of the fiber is 0<B≤30, and the medicine is transdermal The C portion of the absorption promoter accounting for the total weight of the fiber is 0<C≤10, and the drug is one of tamoxifen, isoflavones, bromocriptine and danazol or a combination of more than one; The drug transdermal absorption accelerator is one of azone, oleic acid, N-methyl-2-pyrrolidone, propylene glycol, borneol or a combination of more than one. The invention has a simple manufacturing process and is easy to realize; the source of raw materials is abundant and the cost is low; the bra made of medicinal polyacrylonitrile fiber cloth prevents hyperplasia of mammary glands, which is convenient in medication, has little side effects, and does not affect the external beauty of patients, which is very suitable for modern women Human needs for healing and pursuing beauty.
Owner:义乌市宏光针织有限公司
Orally administered danazol sustained-release capsule and preparation method thereof
InactiveCN102302474APrevent rapid dissolution releasePrevent dissolution releaseOrganic active ingredientsPharmaceutical delivery mechanismMedicineSustained Release Capsule
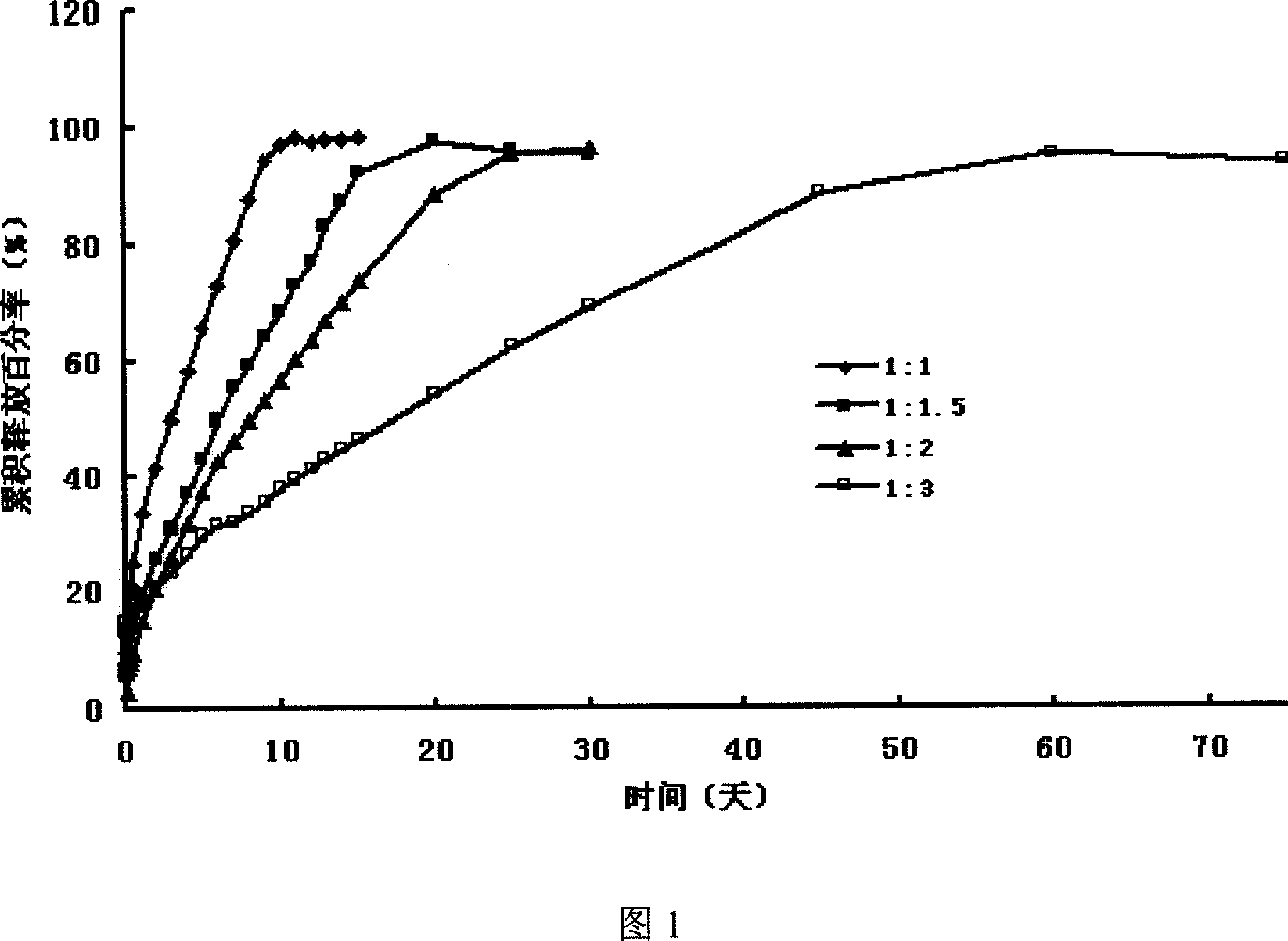
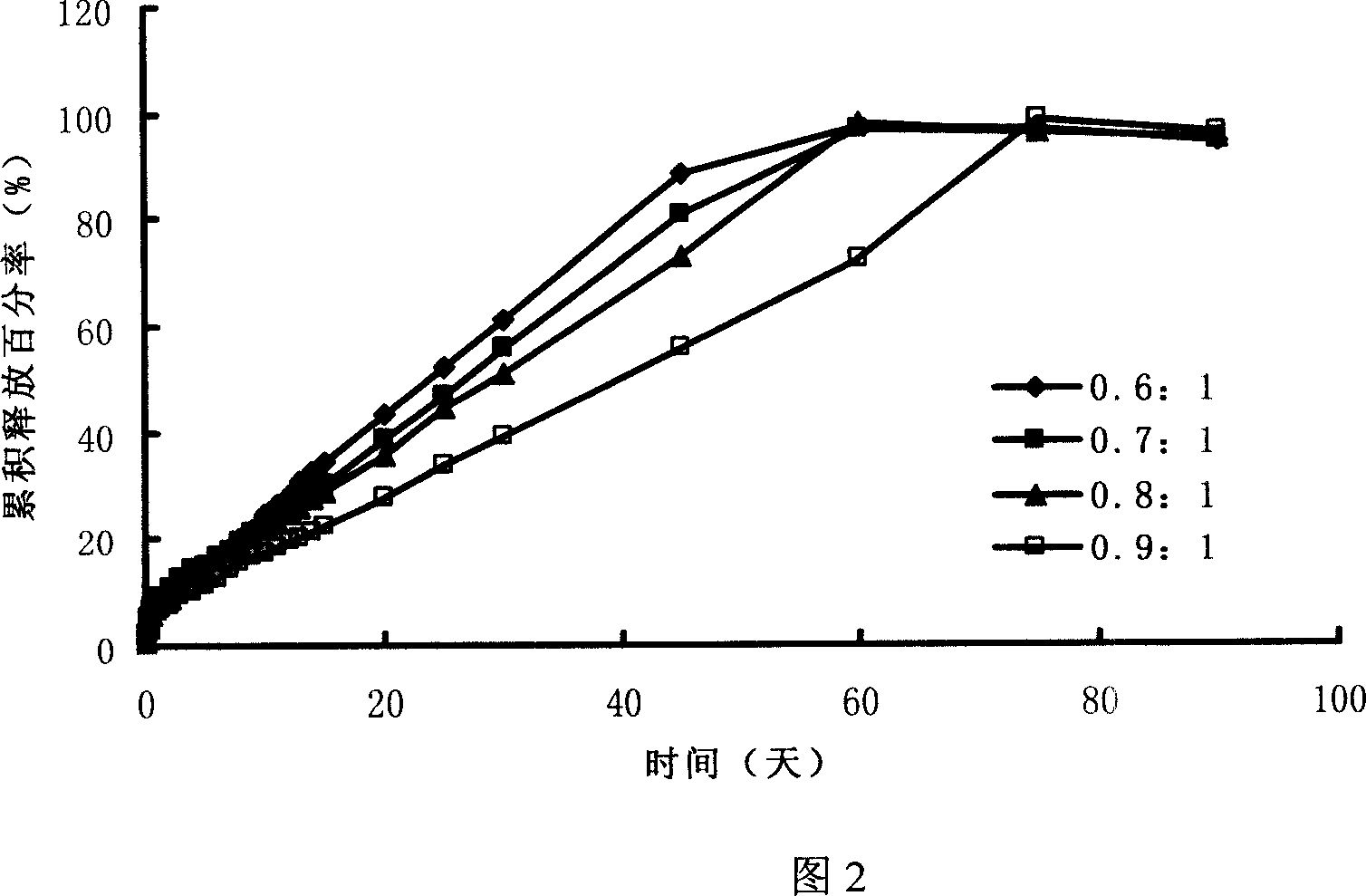
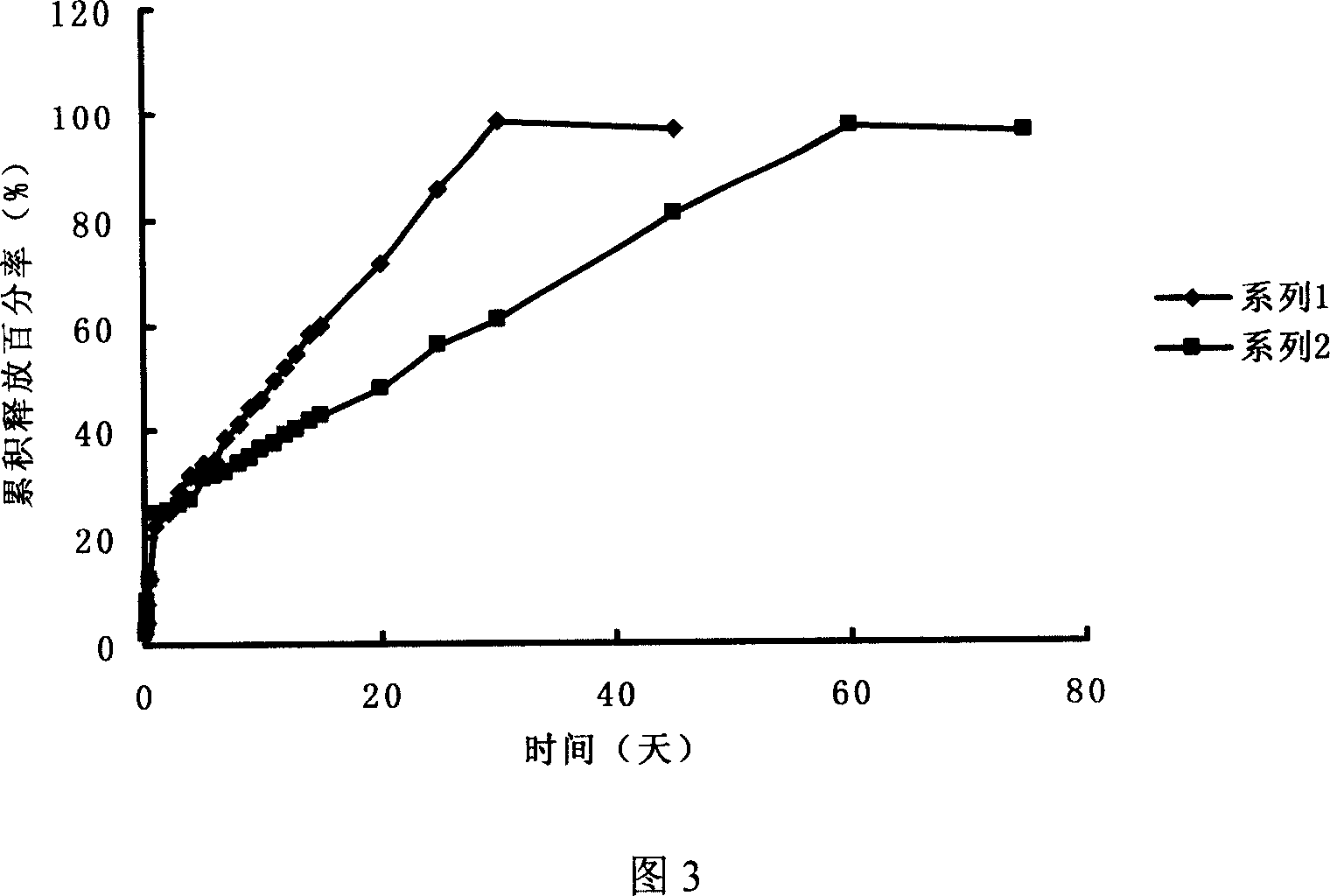
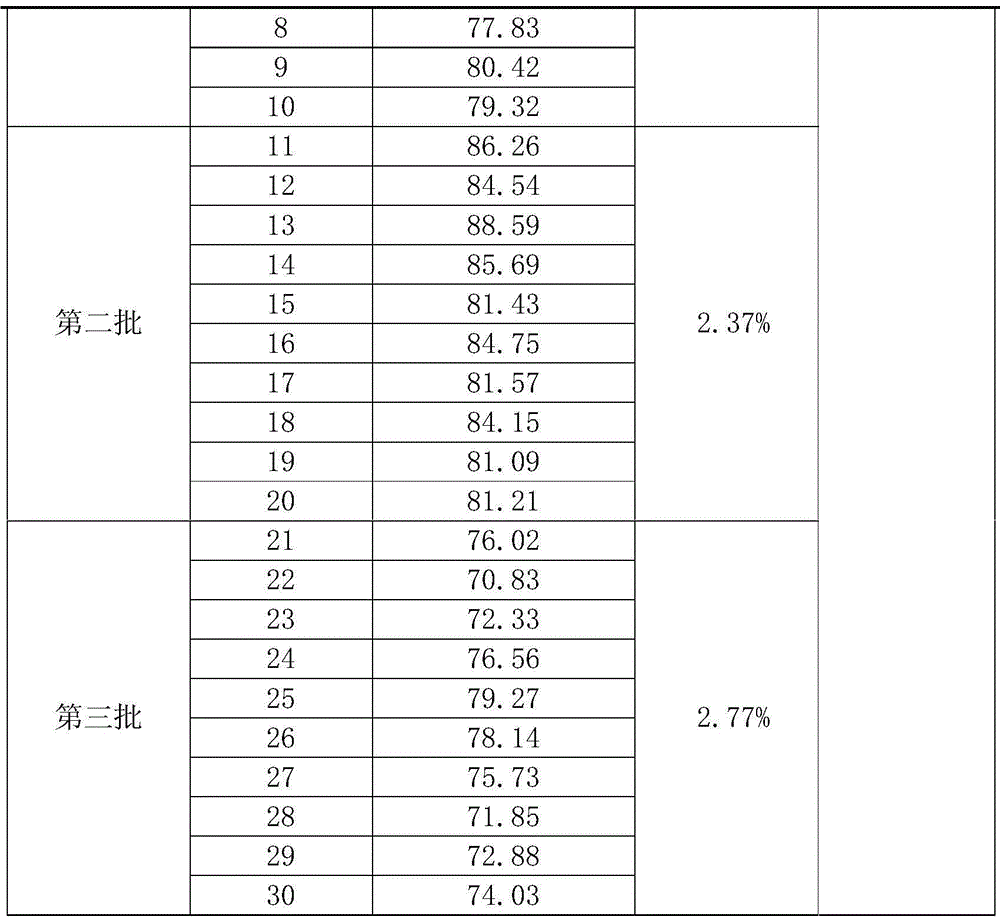
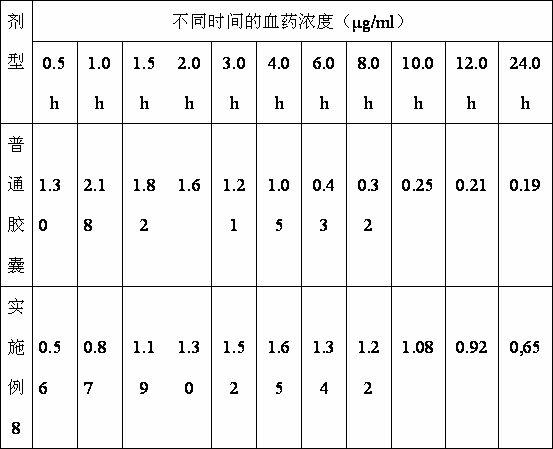
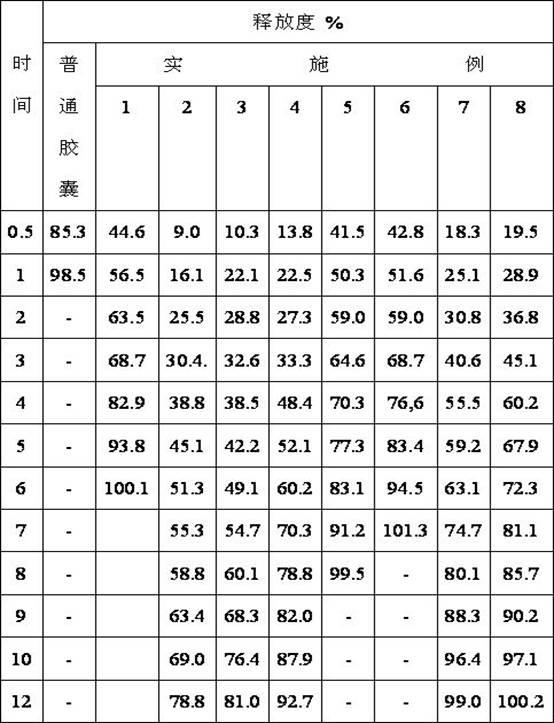
The invention provides an orally administered danazol sustained-release capsule and a preparation method thereof. The orally administered danazol sustained-release capsule mainly comprises danazol and a sustained-release frame material, wherein the mass proportion of the danazol to the sustained-release frame material is 1:(0.32-2.1). The orally administered danazol sustained-release capsule also can comprise a thinning agent and a flow aid. The orally administered danazol sustained-release capsule provided by the invention has steady in-vitro release rate and in-vivo blood concentration, exhibits excellent sustained-release property; when applied in clinic, the administration frequency can be reduced, the orally administered danazol sustained-release capsule is convenient to administer, side effects can be reduced, and the orally administered danazol sustained-release capsule is particularly suitable for patients who take drugs for a long time.
Owner:JIANGSU LIANHUAN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com