Pharmaceutical composition containing danazol and application thereof
A composition, the technology of danazol, which is applied in the field of pharmaceutical composition containing danazol and its preparation, can solve the problems affecting the effectiveness and safety, reducing the compliance of patients taking medicine, and the low content of danazol, and achieves Improve the safety of use, simplify the clinical drug regimen, and facilitate swallowing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
[0034] Examples 1-3 Preparation of Orally Disintegrating Tablets of Danazol
[0035] The composition of danazol oral disintegrating tablet is shown in Table 1, and its preparation method is as follows:
[0036] Pass danazol through a 100-mesh sieve, and other excipients through an 80-mesh sieve; weigh the prescription amount of danazol, mix with the prescription amount of lecithin, sodium lauryl sulfate, sodium carboxymethyl starch, colloidal silicon dioxide, Cross-linked polyvinyl pyrrolidone XL-10 (inside), moistened with 45% ethanol to make soft material; add the above mixture, lactose, low-substituted hydroxypropyl methylcellulose, cross-linked polyvinyl pyrrolidone XL-10 ( outside), mix well, moisten with 45% ethanol to make soft material, granulate; pass through a 40-mesh sieve; add magnesium stearate in the prescribed amount, and press into tablets.
[0037] Table 1 embodiment 1-3 danazol tablet composition
[0038] Composition (mg) Example 1 Example 2 ...
Embodiment 4-6
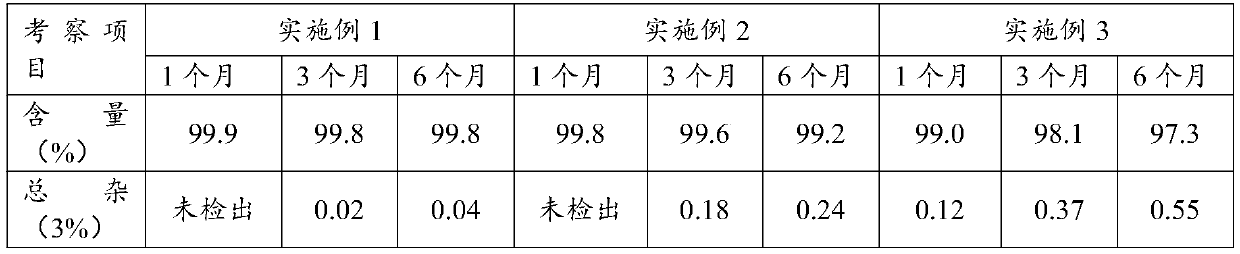
[0039] Examples 4-6 Tablet Properties and Dissolution Investigation
[0040] 1. Tablet performance investigation
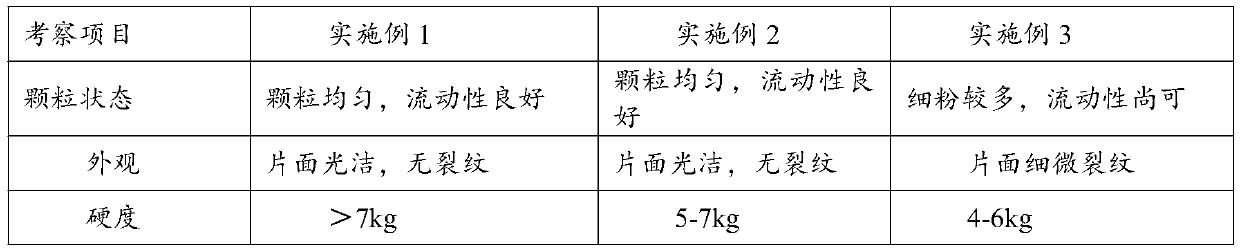
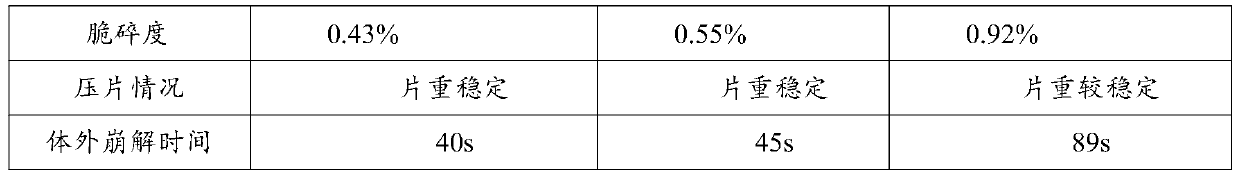
[0041] The properties of the tablets of Examples 1-3 were investigated, and the results are shown in Table 2.
[0042] Table 2 Examples 1-3 Tablet Properties
[0043]
[0044]
[0045] It can be seen from the results in Table 2 that, compared with Example 3, the tablets prepared in Example 1-2 have uniform particles, good fluidity, smooth surface without cracks, and significantly improved hardness and friability. It is not prone to chipping, capping or cracking, and can completely disintegrate in 45s.
[0046] 2. Dissolution determination
[0047]Take this product, according to the dissolution and release determination method (general rule 0931 second method), use 0.1mol / L hydrochloric acid solution-isopropanol (3:2) 1000mL as the dissolution medium, the rotation speed is 80 revolutions per minute, after At 30 minutes, take 25 mL of solution and filter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com