Orally administered danazol sustained-release capsule and preparation method thereof
A technology of danazol and capsules, which is applied in the field of medicine, can solve the problems of affecting the therapeutic effect, long medication cycle, missed doses, and wrong doses, and achieve safe and effective treatment, easy to purchase in large quantities, and prevent rapid dissolution and release.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
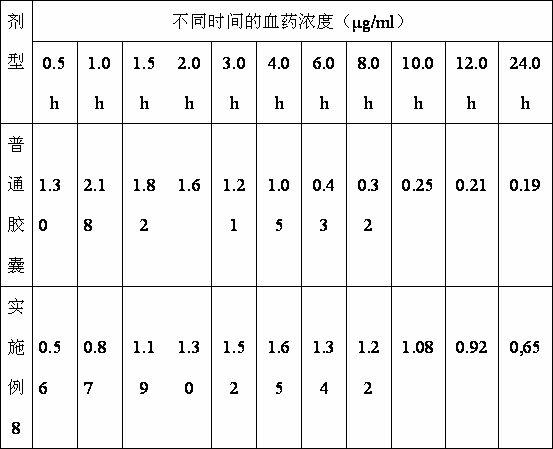
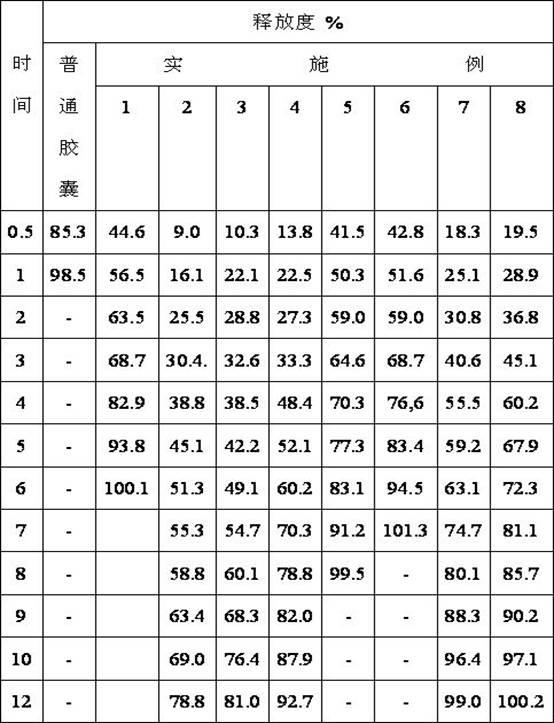
Image
Examples
Embodiment 1
[0016] Prescription (by weight)
[0017] Danazol 26.9 parts
[0018] Hypromellose K4M 8.1 parts
[0019] Microcrystalline cellulose 26.9 parts
[0020] Acrylic No. 3 resin 5.4 parts
[0021] Mannitol 32.3 parts
[0022] Magnesium stearate 0.54 parts
[0023] method
[0024] Weigh danazol, hypromellose K4M, microcrystalline cellulose, acrylic acid No. 3 resin, and mannitol according to the above-mentioned prescription ratio, mix evenly in equal increments, pass through a 200-mesh sieve, and mix with 8% polyvinylpyrrolidone K25 ethanol liquid is made into soft material, granulated with 20-mesh sieve, dried at 50-60°C, added with magnesium stearate, packed in capsules.
Embodiment 2
[0026] Prescription (by weight)
[0027] Danazol 40.6 parts
[0028] Hypromellose E15 4.06 parts
[0029] Ethyl cellulose 20.3 parts
[0030] Cellulose acetate phthalate 4.06 parts
[0031] Lactose 30.5 parts
[0032] Magnesium stearate 0.4 parts
[0033] method
[0034] Weigh danazol, hypromellose E15, ethyl cellulose, cellulose acetate phthalate, and lactose according to the prescription ratio, mix them in equal increments, pass through a 200-mesh sieve, and mix them with 10% starch slurry Made into soft material, granulated with 20-mesh sieve, dried at 50-60°C, added with magnesium stearate, packed in capsules.
Embodiment 3
[0036] Prescription (by weight)
[0037] Danazol 42.4 parts
[0038] Hypromellose K10M 4.24 parts
[0039] Sodium carboxymethylcellulose 4.24 parts
[0040] Ethyl cellulose 21.2 parts
[0041] Mannitol 27.6 parts
[0042] Micropowder silica gel 0.42 parts
[0043] method
[0044] Weigh danazol, hypromellose K10M, sodium carboxymethyl cellulose, ethyl cellulose and mannitol respectively according to the prescription ratio, mix them in equal increments, pass through a 200-mesh sieve, and use 10% starch slurry Made into soft materials, granulated with a 20-mesh sieve, dried at 50-60°C, added with micronized silica gel, and packed in capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com