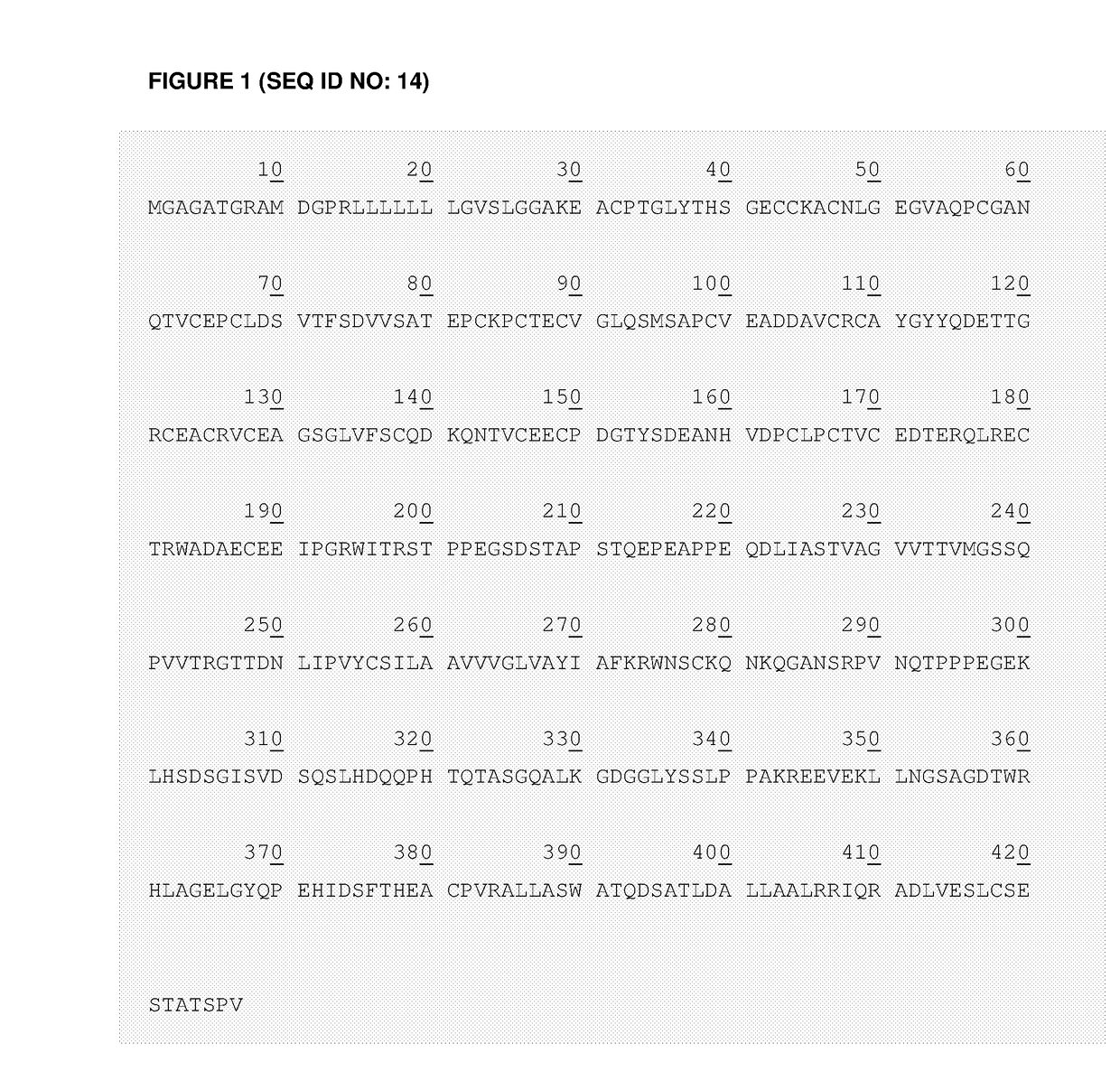
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "Tumour antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
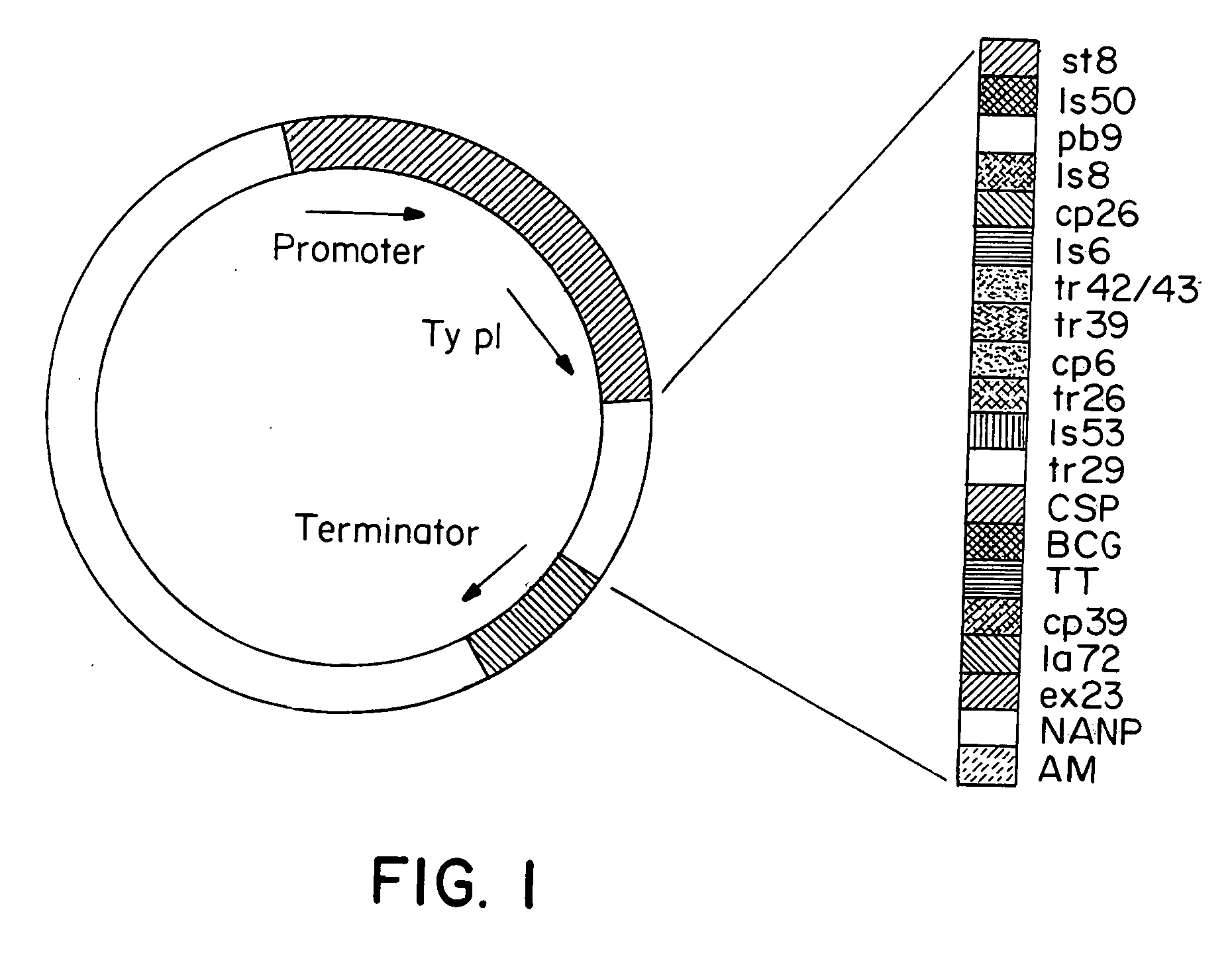
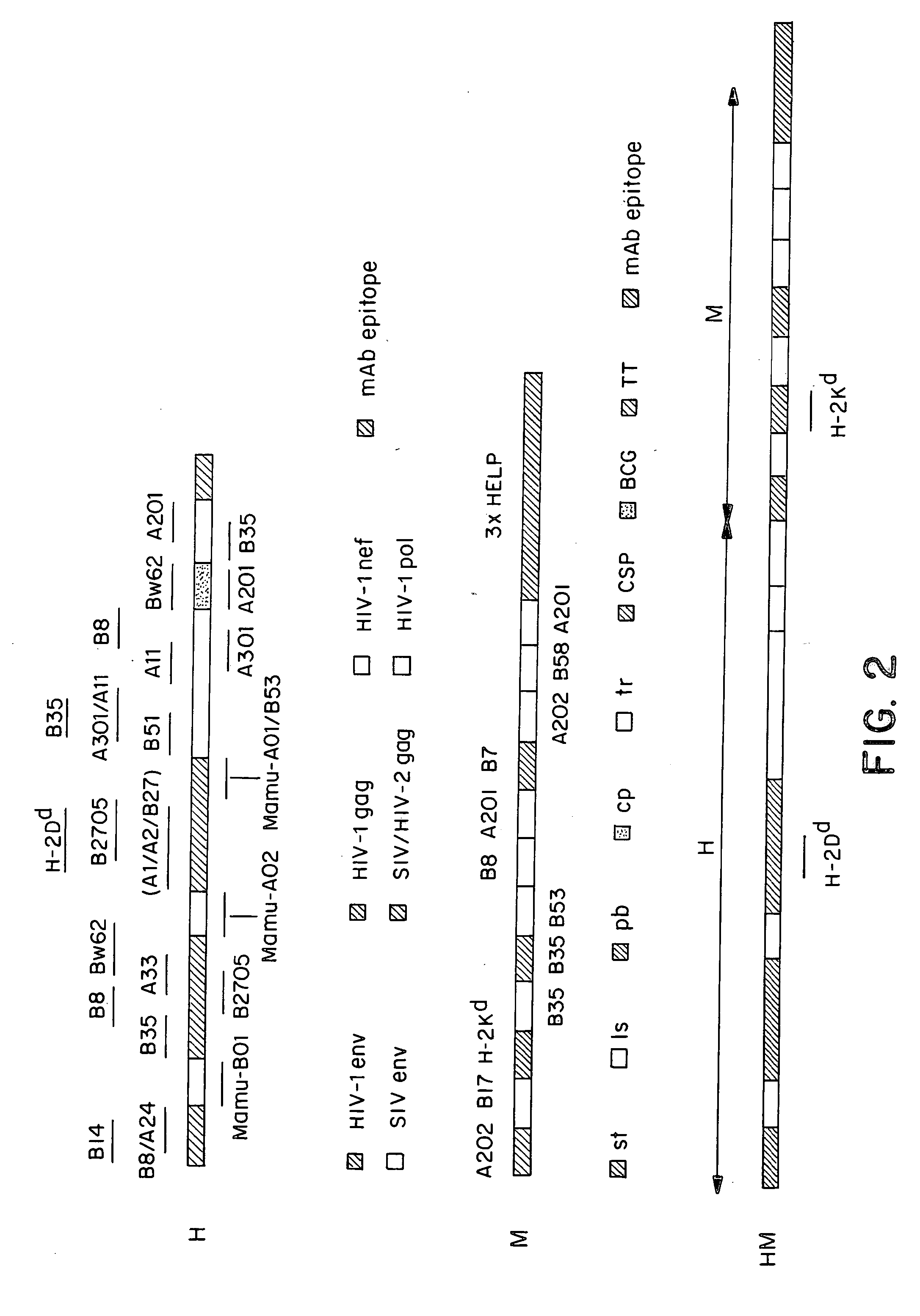
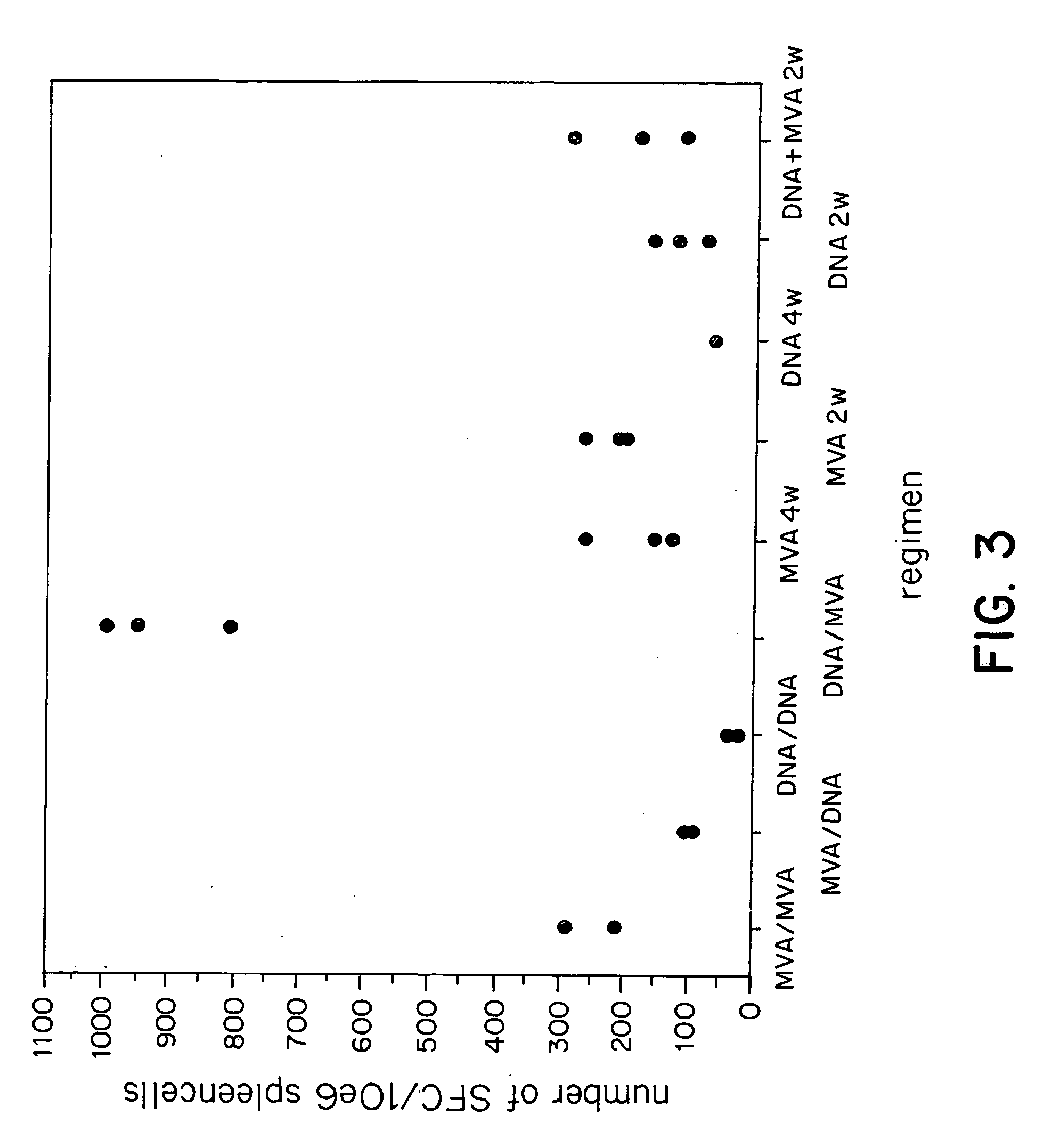
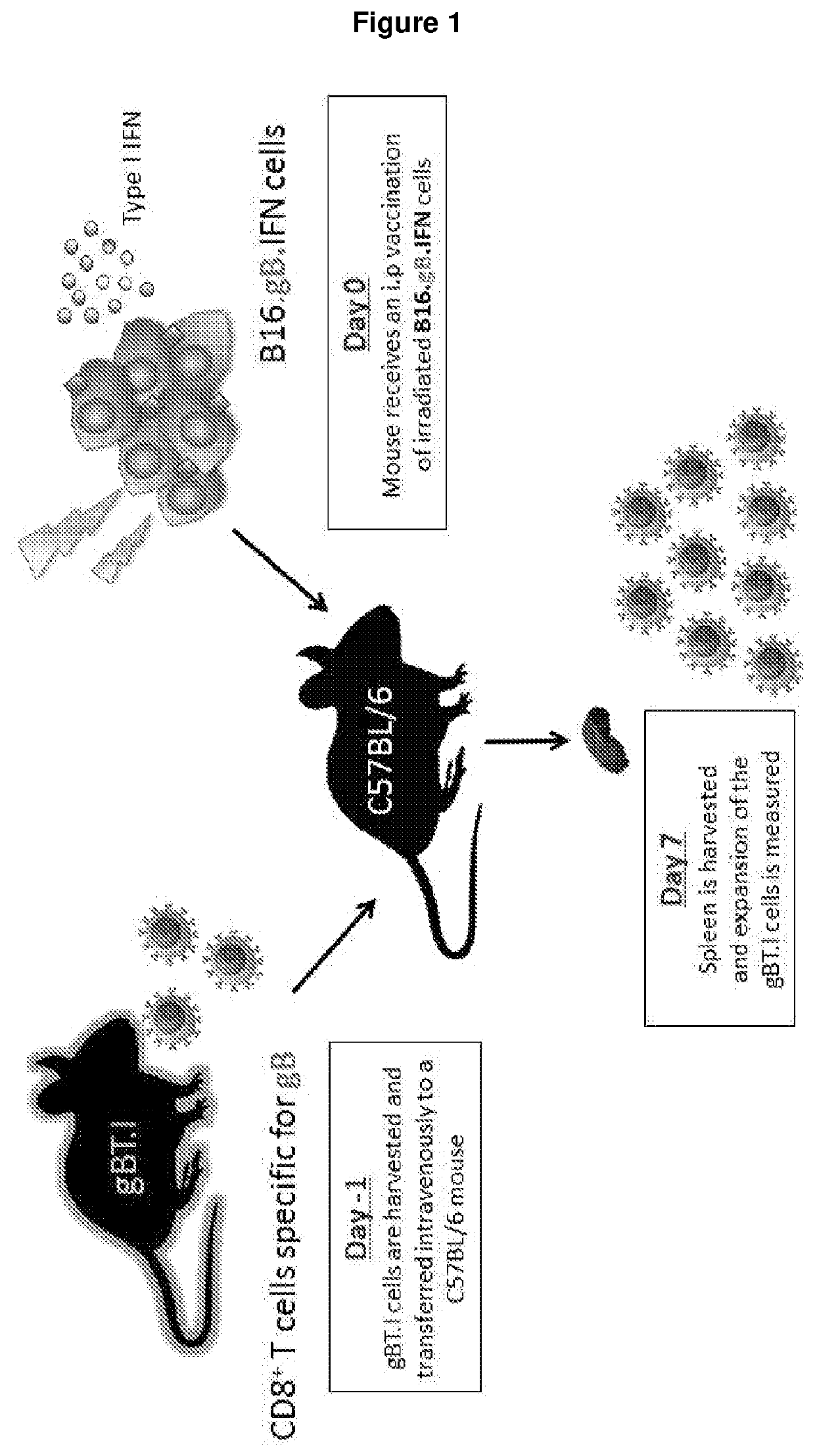
Methods and reagents for vaccination which generate a CD8 T cell immune response
New methods and reagents for vaccination are described which generate a CD8 T cell immune response against malarial and other antigens such as viral and tumour antigens. Novel vaccination regimes are described which employ a priming composition and a boosting composition, the boosting composition comprising a non-replicating or replication-impaired pox virus vector carrying at least one CD8 T cell epitope which is also present in the priming composition.
Owner:OXXON THERAPEUTICS LTD
Immunotherapy
InactiveUS20030194804A1Inhibit T cell responseInhibitory responseBiocidePeptide/protein ingredientsNucleic acid sequencingWilms' tumor
A method is provided for enhancing the reactivity of a T cell toward a tumour cell which method comprises: (a) isolating a T cell which is a tumour infiltrating lymphocyte (TIL) from a patient having said tumour cell present in their body; (b) introducing a nucleic acid sequence into the TIL, which sequence is capable of inhibiting or preventing expression of an endogenous Notch ligand in the TIL; and (c) re-introducing the transfected TIL into the patient; wherein the T cell comprises a T cell receptor specific for a tumour antigen expressed by the tumour cell.
Owner:CELLDEX THERAPEUTICS LIMITED
NY-ESO-1 tumour antigen mimic epitope and use thereof
InactiveCN101381402AImproving immunogenicityStrong specificityPeptidesAntibody medical ingredientsCtl epitopePredictive methods
The invention discloses epitope for an NY-ESO-1 tumor antigen. The amino acid sequence of the mimic epitope is Ser-Leu-Leu- Met-Phe-Ile-Thr-Trp-Cys, namely SLLMFITWC; the mimic epitope can raise a CTL immunity response, can undergo a cross reaction with a natural epitope, has the characteristics of strong immunogenicity, immune tolerance breaking, strong specificity, safety, low cost, and easy synthesis and storage, and can be used to prepare tumor-therapeutic polypeptide vaccine. The invention also discloses a method for predicting the mimic epitope, which comprises steps of the establishment of a structural model of a TCR-pMHC complex, the analysis of TCR binding sites of the epitope, and the analysis of amino acid replacement of the TCR binding sites of the epitope. The method is also applicable to the computer-aided modification of CTL epitopes of other antigens, and can provide a useful tool for the design and research of therapeutic polypeptide vaccine.
Owner:ARMY MEDICAL UNIV
Vaccination methods
ActiveUS20120014990A1SsRNA viruses negative-senseOrganic active ingredientsVaccinationPre existing immunity
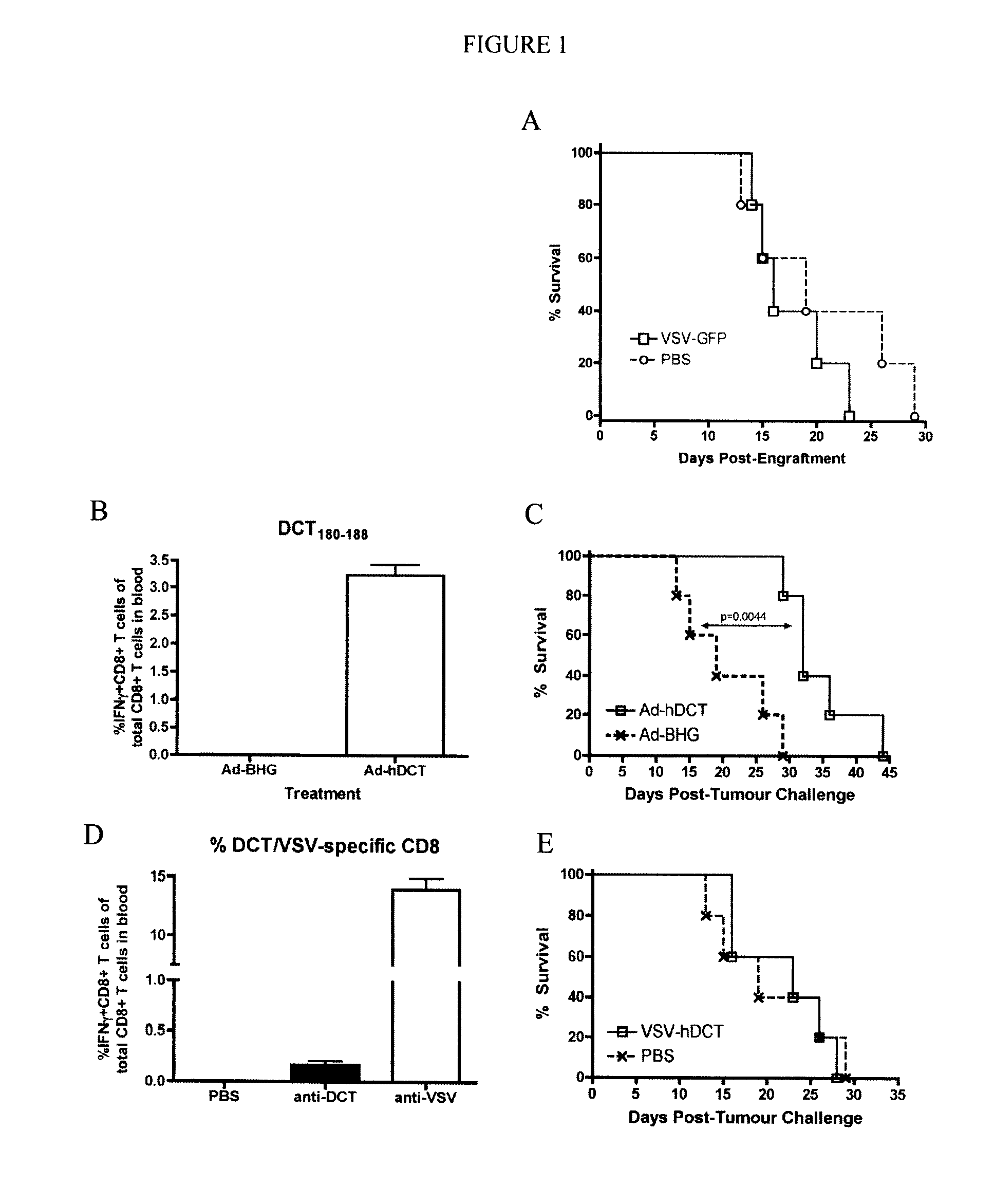
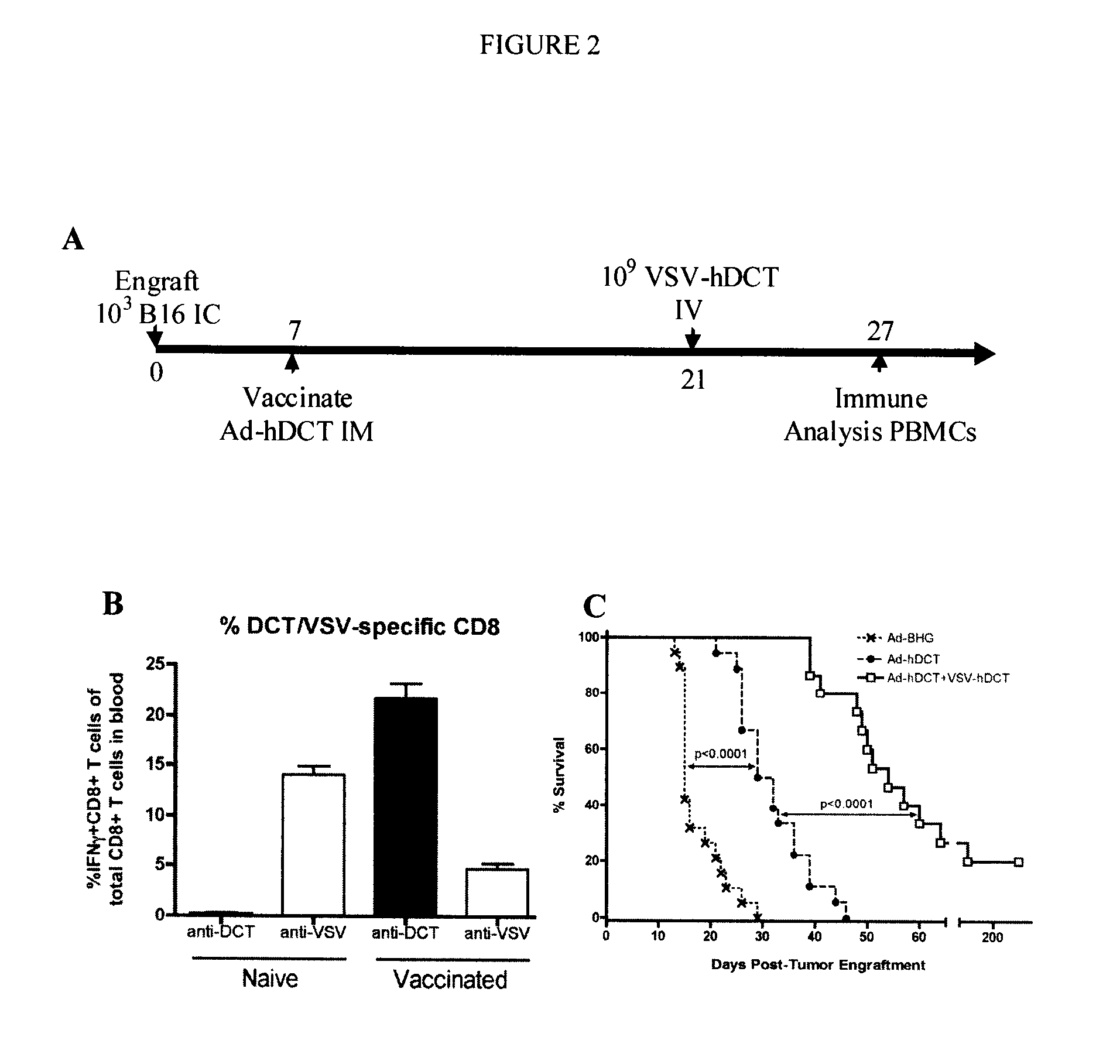
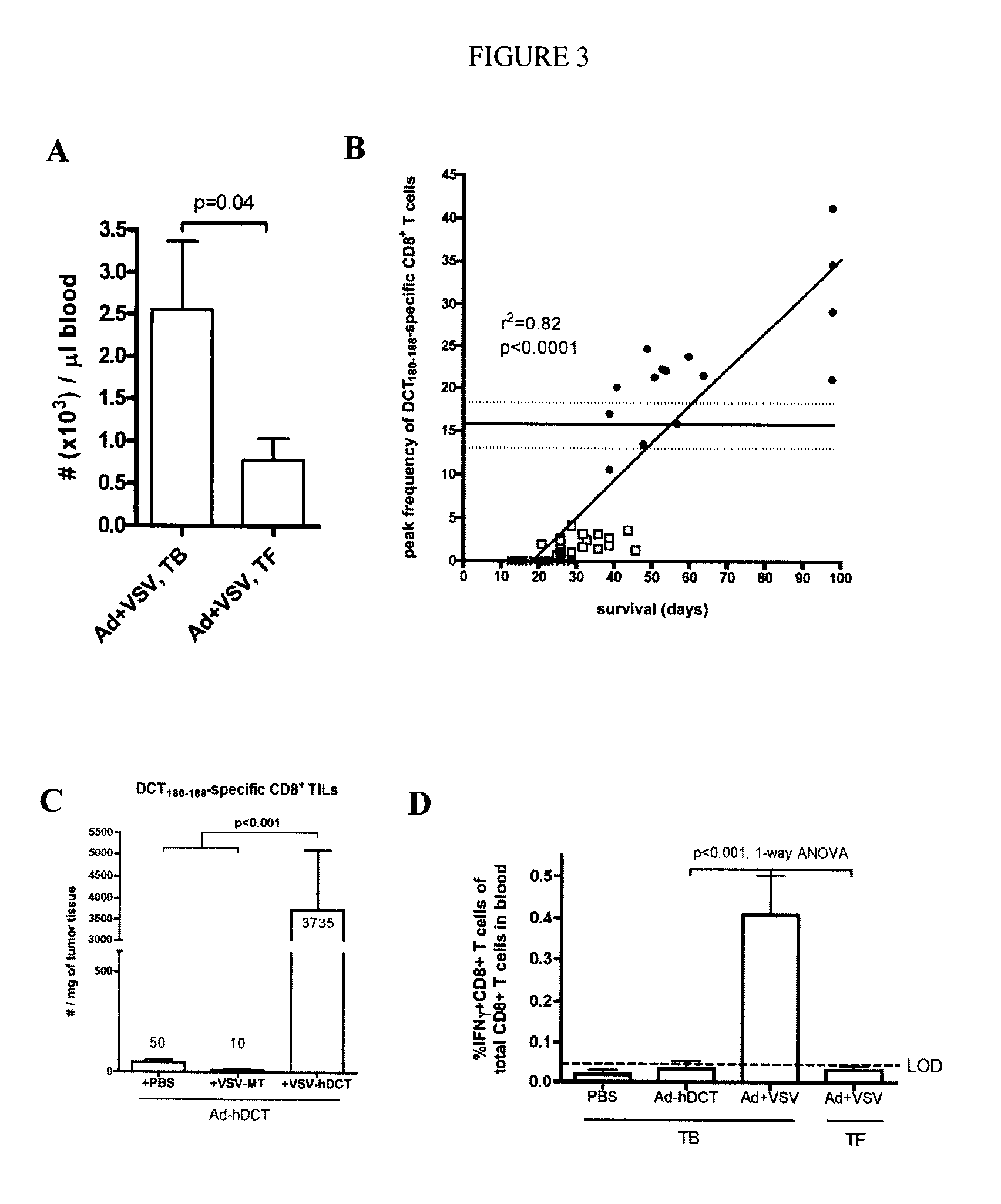
In one aspect, a method of treating cancer in a mammal is provided. The method comprises administering to the mammal an oncolytic vector that expresses a tumour antigen to which the mammal has a pre-existing immunity. In another aspect, a method of boosting immune response in a mammal having a pre-existing immunity to an antigen is provided comprising intravenous administration to the mammal of a B-cell infecting vector that expresses the antigen.
Owner:TURNSTONE LLP
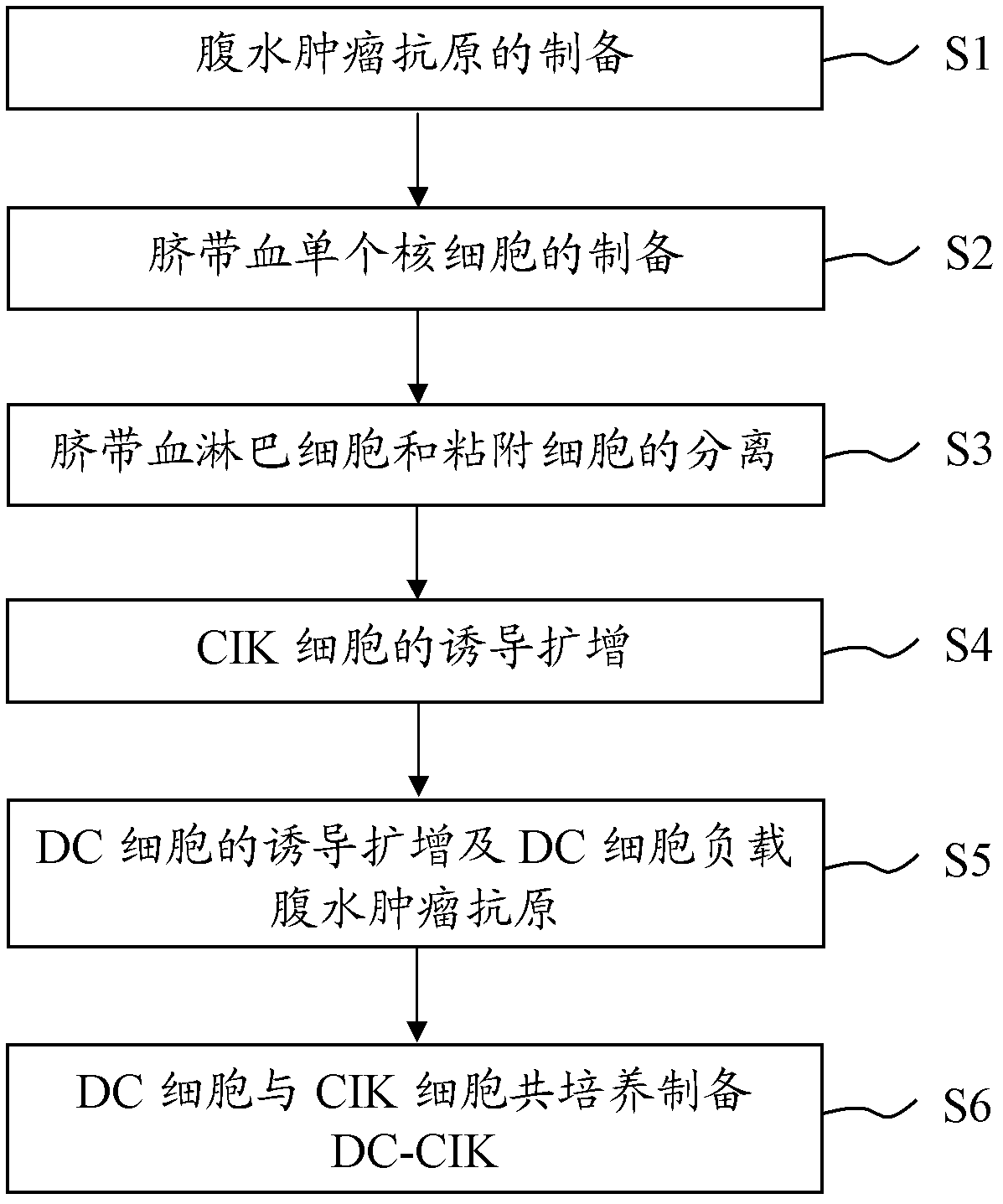
Preparation method of ascites tumor cell sensitized DC-CIK
ActiveCN102321581AHigh activityImprove proliferation efficiencyBlood/immune system cellsAscites tumorsCord blood stem cell
The invention provides a preparation method of ascites tumor cell sensitized DC-CIK and application of the DC-CIK. The preparation method of ascites tumor cell sensitized DC-CIK comprises steps of: preparation of ascites tumour antigen; preparation of cord blood mononuclear cells; separation of cord blood lymphocytes and adherent cells; induction and amplification of the CIK cells; induction and amplification of the DC cells and loading of the ascites tumour antigen by the DC cells; and preparation of DC-CIK through co-culture of DC cells and CIK cells. The DC-CIK prepared by the preparation method of ascites tumor cell sensitized DC-CIK of the invention has high proliferative activity and tumor killing activity. The method has simple operations, easily controlled conditions and low equipment requirements.
Owner:广东省医学医疗有限公司
Bi-function ligand targeting dendritic cell tumor vaccine and preparation method thereof
InactiveCN104623646AReduced clearance rateExtended stayPharmaceutical non-active ingredientsAntibody medical ingredientsDendritic cellT lymphocyte
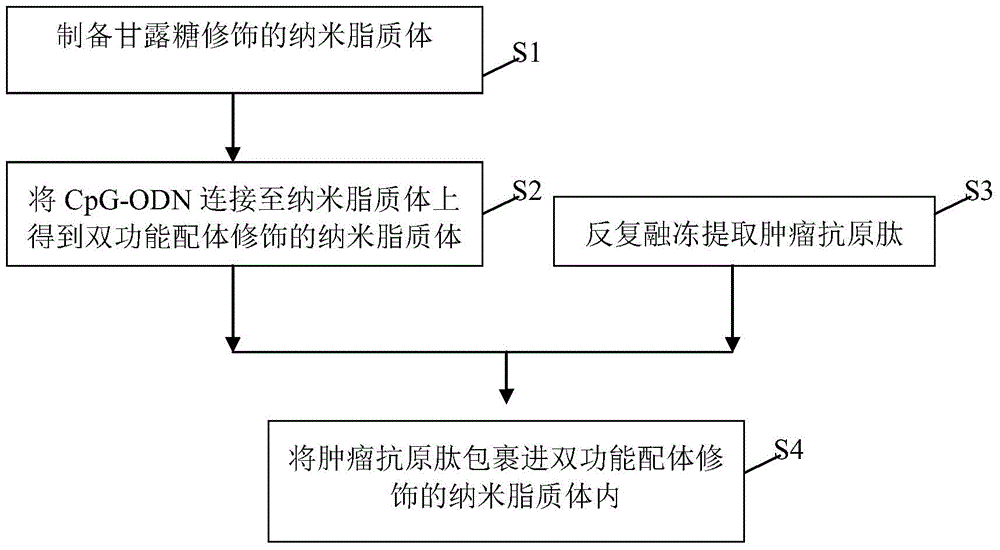
The invention relates to a bi-function ligand targeting dendritic cell tumor vaccine and a preparation method thereof. The method comprises the following steps: S1)preparing mannose modified nanoliposomes; S2)connecting CpG-ODN to the nanoliposomes to obtain the bi-function ligand modified nanoliposomes; S3)extracting tumour antigen peptide from tumor cells by a repeated thawing-freezing method; and S4) coating tumour antigen peptide by the ligand modified nanoliposomes by a freeze-drying-rehydration method. According to the invention, the bi-function ligand modified nanoliposomes can be taken as a carrier, mannose molecules on surface of liposome is specifically targeted to dendritic cells, CpG-ODN is combined to a Toll acceptor 9 in the dendritic cells for promoting the maturation of dendritic cells, matured dendritic cells enables high efficiency presentation of tumour antigen peptide released from inner part of nanoliposomes, T lymphocyte can be activated, and specific antineoplastic effect is generated.
Owner:GUANGXI MEDICAL UNIVERSITY
Preparation method of human dendritic cell tumour vaccine
InactiveCN104491853AHigh purityPromote maturityAntibody medical ingredientsAntineoplastic agentsDendritic cellCell engineering
The invention provides a preparation method of a human dendritic cell tumour vaccine and belongs to the technical field of cell engineering. The human dendritic cell tumour vaccine is characterized in that a DC maturity acceleration agent is added in a process that the dendritic cell is matured from being unmatured, and the DC maturity acceleration agent comprises OK-432 and a gp100 combined DC maturity acceleration agent. By adopting the human dendritic cell tumour vaccine, cancer patients can be treated or high risk of people suffering from cancer can be avoided. The human dendritic cell tumour vaccine prepared by adopting the preparation method provided by the invention has the advantages that DC purity and maturity of the human dendritic cell tumour vaccine are greatly improved, fusion of DC and a tumour antigen is promoted, and treatment of cancer can be facilitated; meanwhile, a preparation technology is simple, and cost is low.
Owner:深圳市赛欧细胞技术有限公司
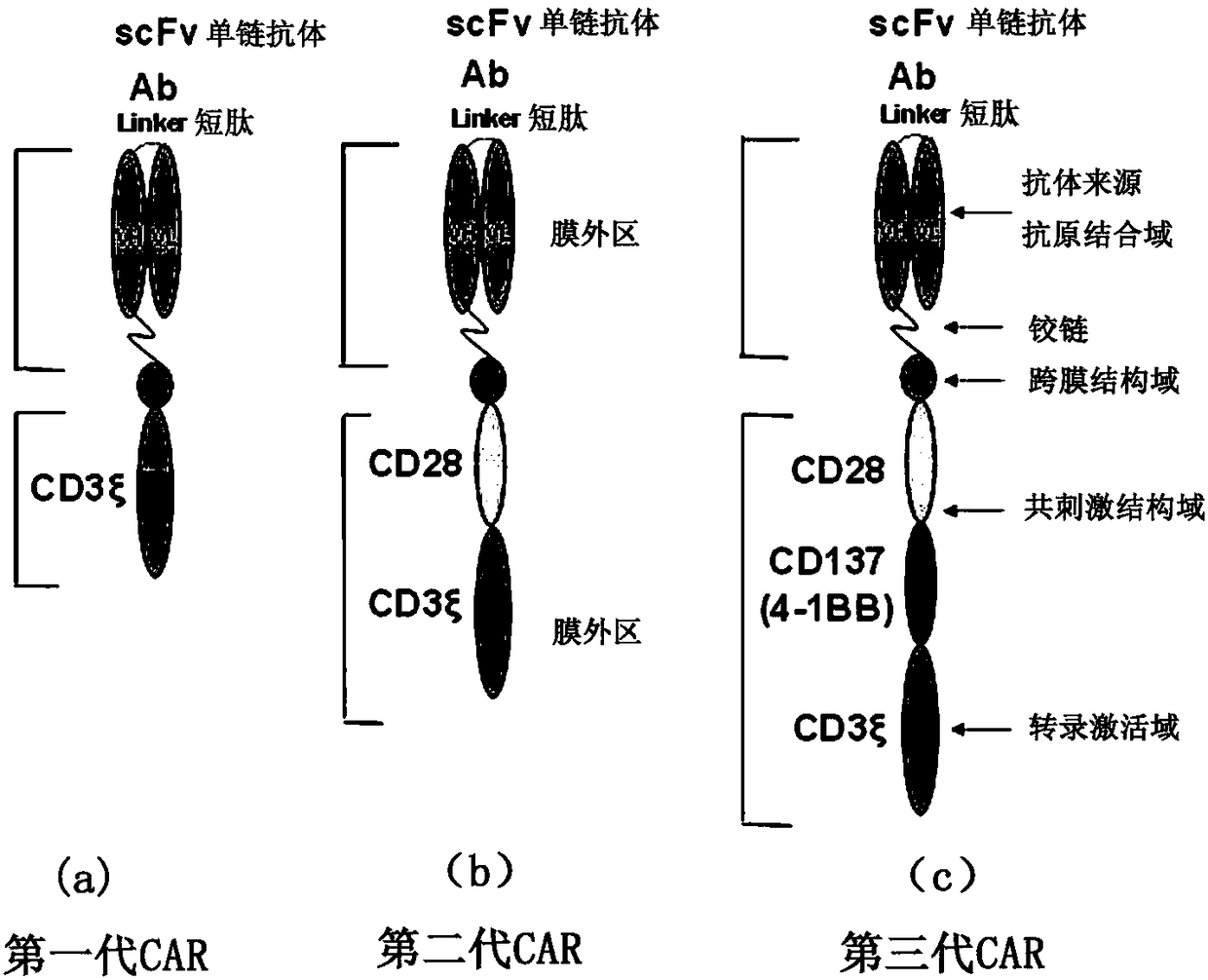
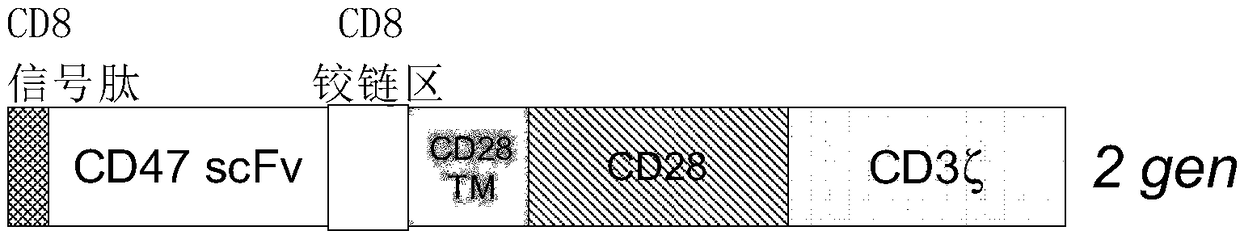
Cd47-car-t cells
ActiveCN108424461AMammal material medical ingredientsImmunoglobulinsAntigen receptorTransmembrane domain
The present invention provides a chimeric antigen receptor (CAR) fusion protein comprising from N-terminus to C-terminus: (i) a single-chain variable fragment (scFv) comprising VH and VL, wherein scFvhas an activity against CD47, (ii) a transmembrane domain, (iii) at least one co-stimulatory domains, and (iv) an activating domain. In one embodiment, the scFv is derived from a humanized anti-CD47antibody. The present invention also provides T cells modified to express the CAR of the present invention.
Owner:GRACELL BIOTECH SHANGHAI CO LTD
Complex of prostate stem cell antigen polypeptide and nucleic acid and its preparation method and application
InactiveCN102294025AFree from degradationImproving immunogenicityGenetic material ingredientsPharmaceutical non-active ingredientsT lymphocytePeptide vaccine
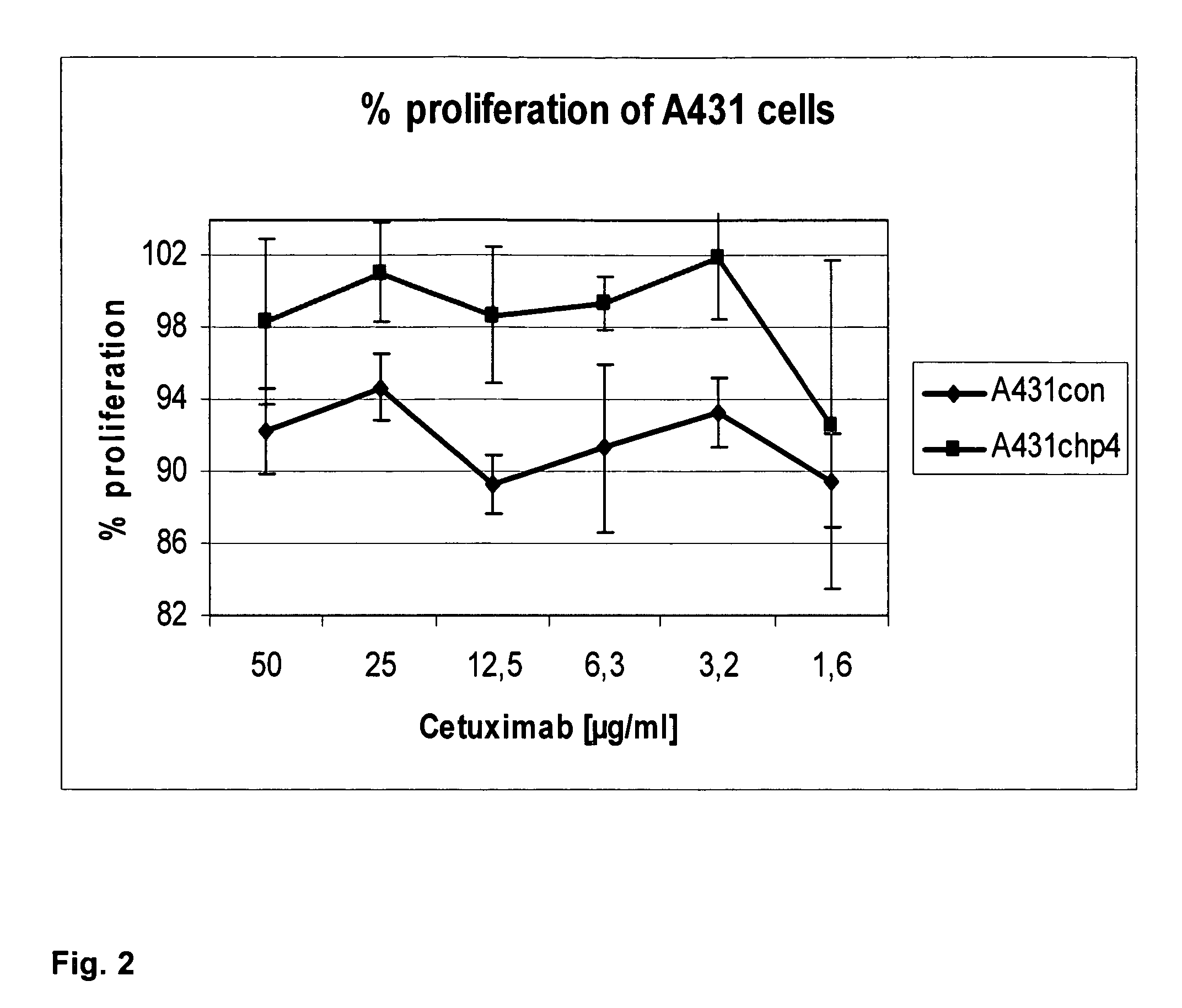
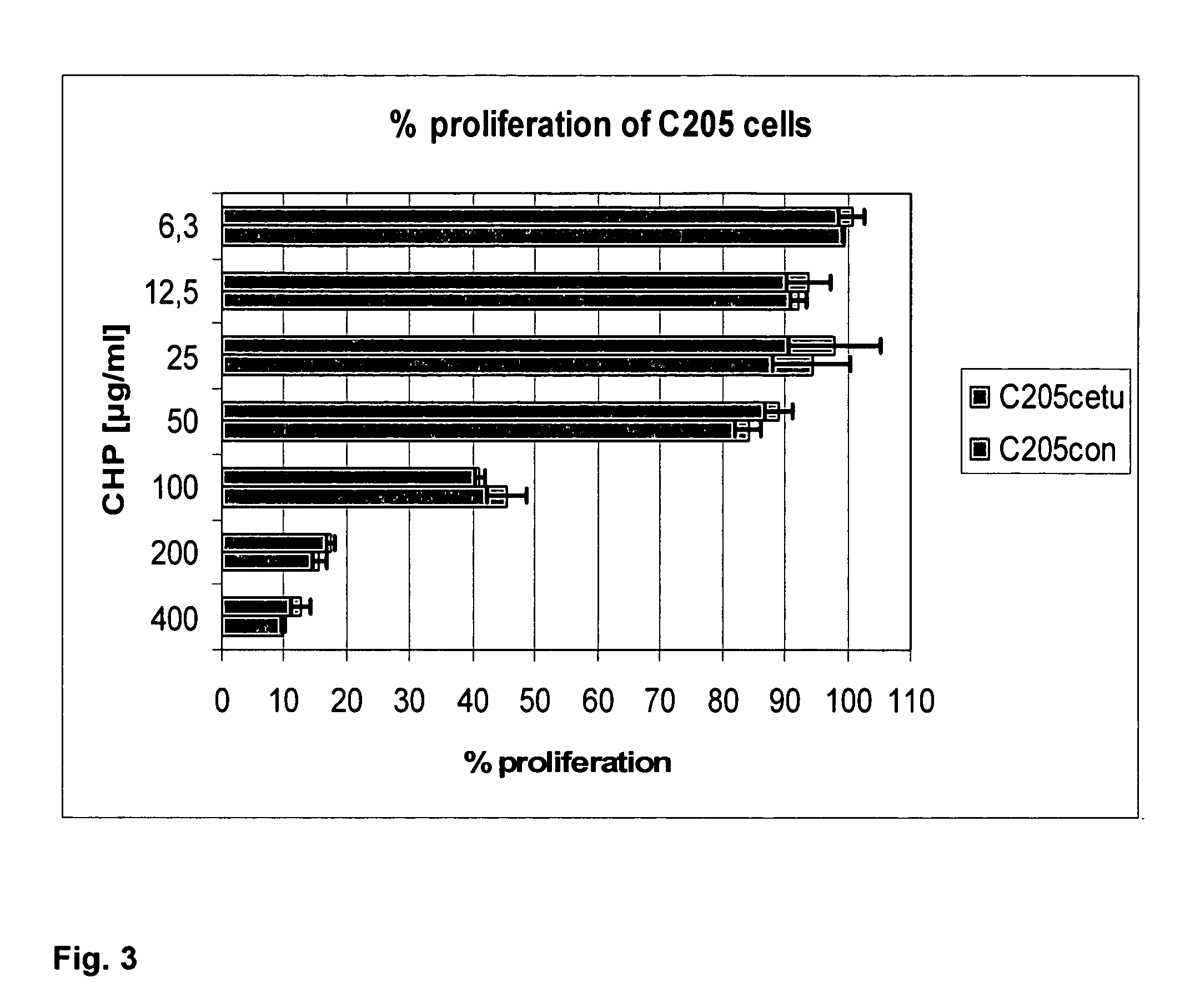
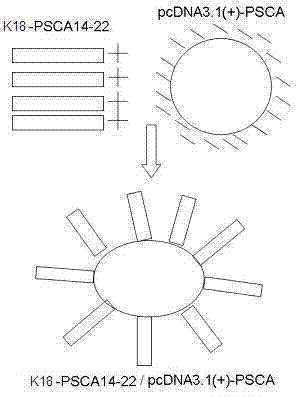
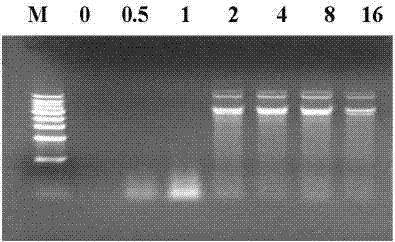
The invention discloses a compound of PSCA (Prostate Stem Cell Antigen) polypeptide and nucleic acid, which is a granular compound obtained by the way that through positive and negative charge attraction, a PSCA gene recombination eukaryotic expression vector is packaged by fusogenic peptide formed by a positive ion peptide DNA (Deoxyribonucleic Acid) transport carrier and HLA-A2 limit CTL (Cytotoxic T Lymphocyte) epitope peptide PSCA 14-22. The preparation method comprises the steps that in buffer salt solution with pH of 7.2-7.6, the fusogenic peptide and the PSCA gene recombination eukaryotic expression vector are added according to a certain positive and negative charge ratio, are then mixed, and are left standing under room temperature for 0.5-1.5 hours to obtain the compound; the compound is not only combined with the advantages of DNA vaccine and epitope peptide vaccine, but also can imitate natural virus to directly 'infect' APC (Antigen Presenting Cell) and other immune cells, so as to stimulate strong CTL response in vivo. The compound is expected to break down selftolerance of tumour antigen, can be used to prepare prostate cancer therapeutic vaccine of more than 50 % of population in China, and has potential and good development and application prospect in the prostate cancer immunization therapy field.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
Specific biomarker set for non-invasive diagnosis of liver cancer
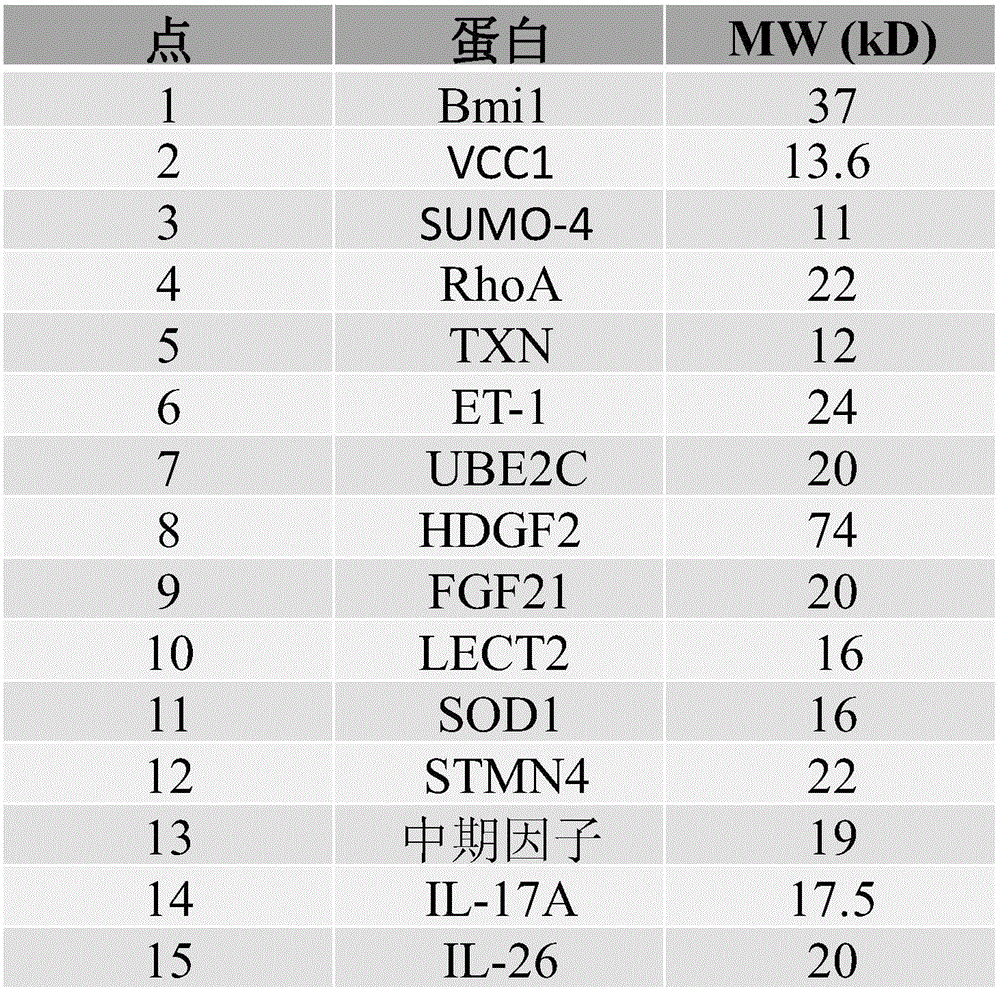
Cells within liver tumour mass comprise a unique set of proteins / tumour antigens when compared to the normal liver tissues epithelial cells juxtaposed to the tumour. The presence of tumour antigens couples the production of auto-antibodies against these tumour antigens. The present invention relates to the identification and elucidation of a protein set that can act as a novel marker set for liver cancer diagnosis and prognosis. Specifically, it relates to a kit that enables diagnostic and prognostic measurement of auto-antibodies in serum of liver cancer patients. The present invention provides a non-invasive, specific, sensitive, and cost effective detection and quantification method by evaluating a set of validated liver cancer proteins / tumour antigens, which includes Bmi-1, VCC1, SUMO-4, RhoA, TXN, ET-1, UBE2C, HDGF2, FGF21, LECT2, SOD1, STMN4, Midkine, IL-17A or IL26, to complement the conventional diagnostic methods.
Owner:DRAGON VICTORY DEV
Novel Adenovirus Vectors
ActiveUS20120058138A1Enhances adenoviral vector immunogenicityEnhances protective efficacyEnzymologyImmunoglobulinsNucleic acid sequencingFhit gene
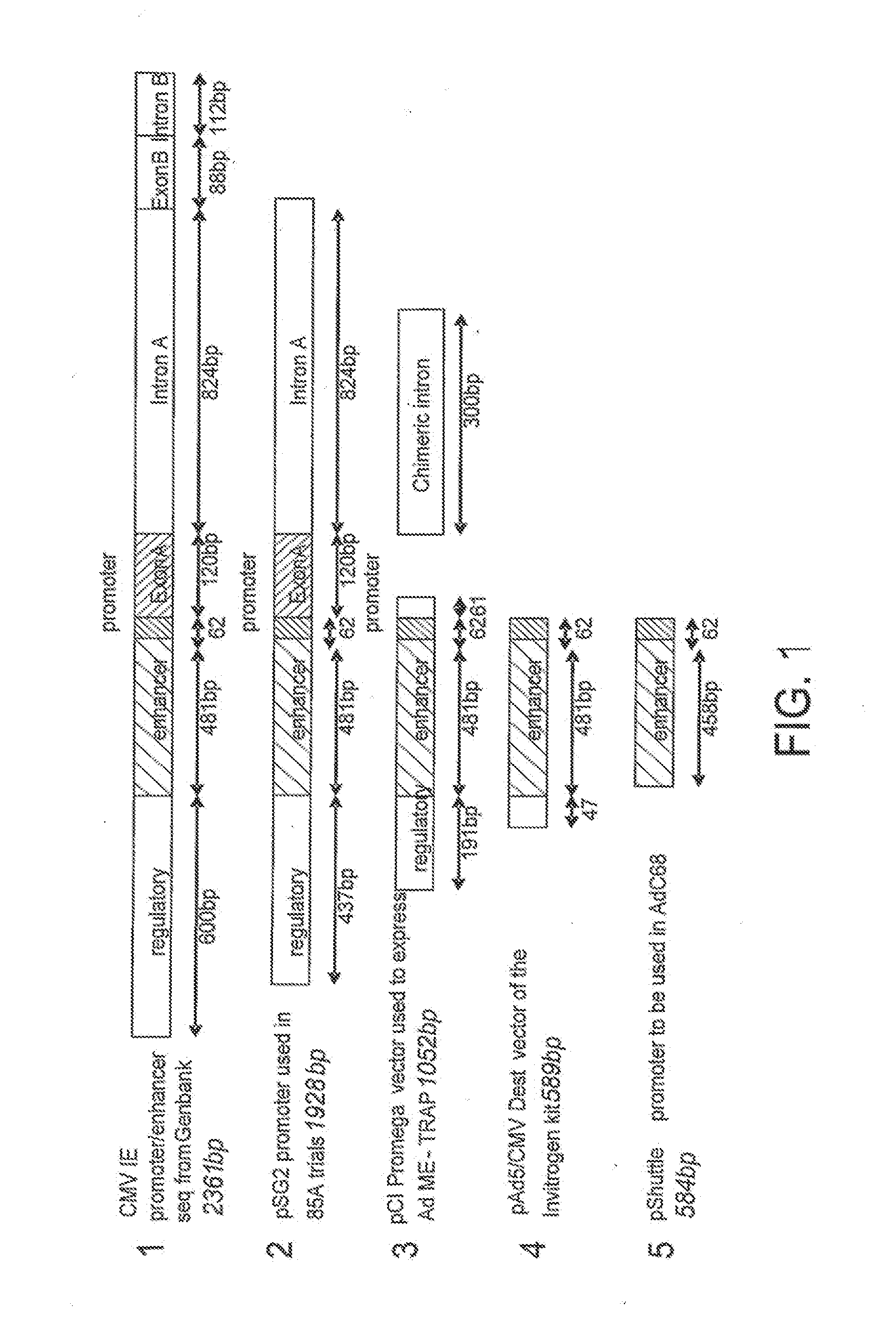
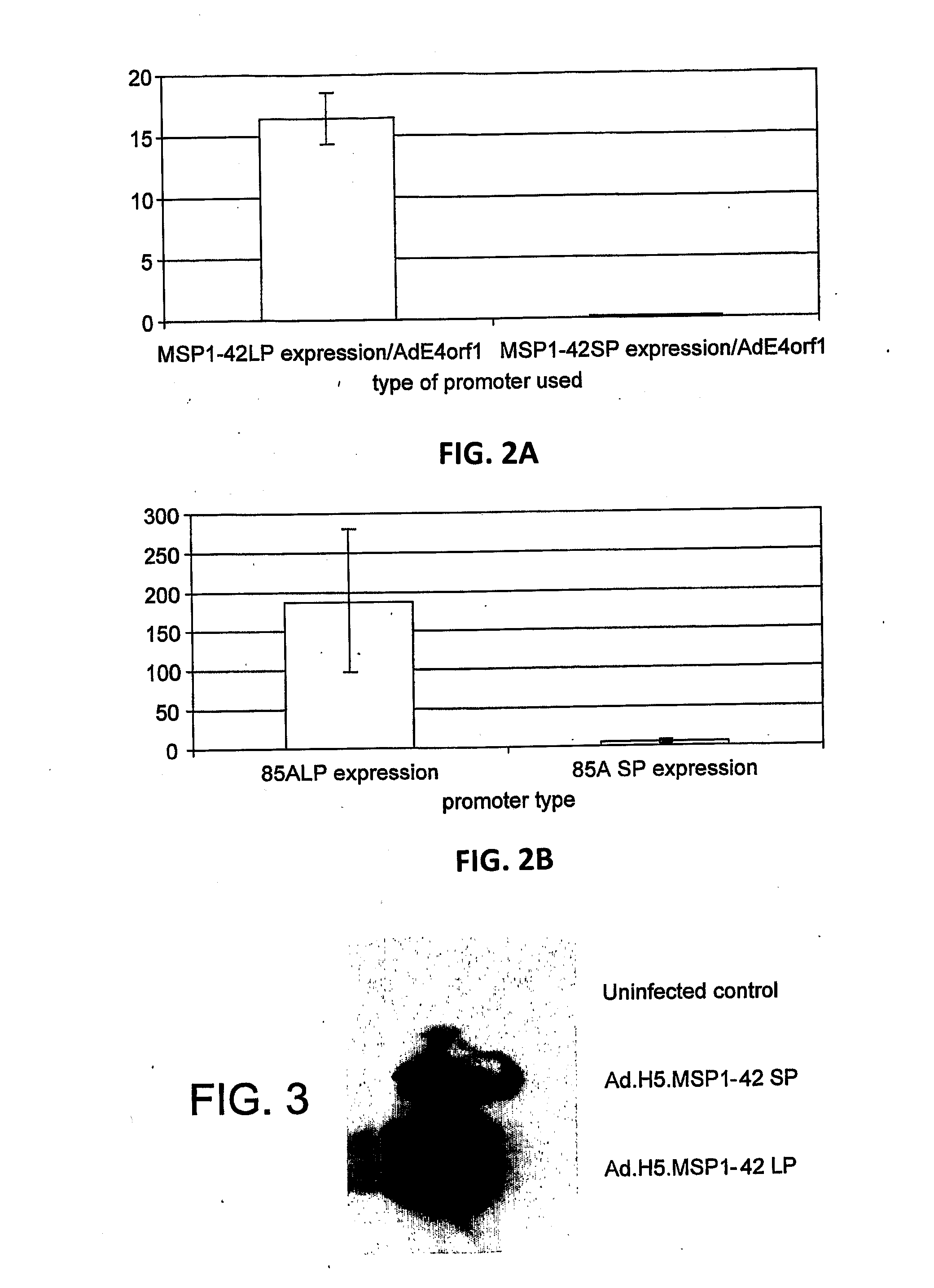
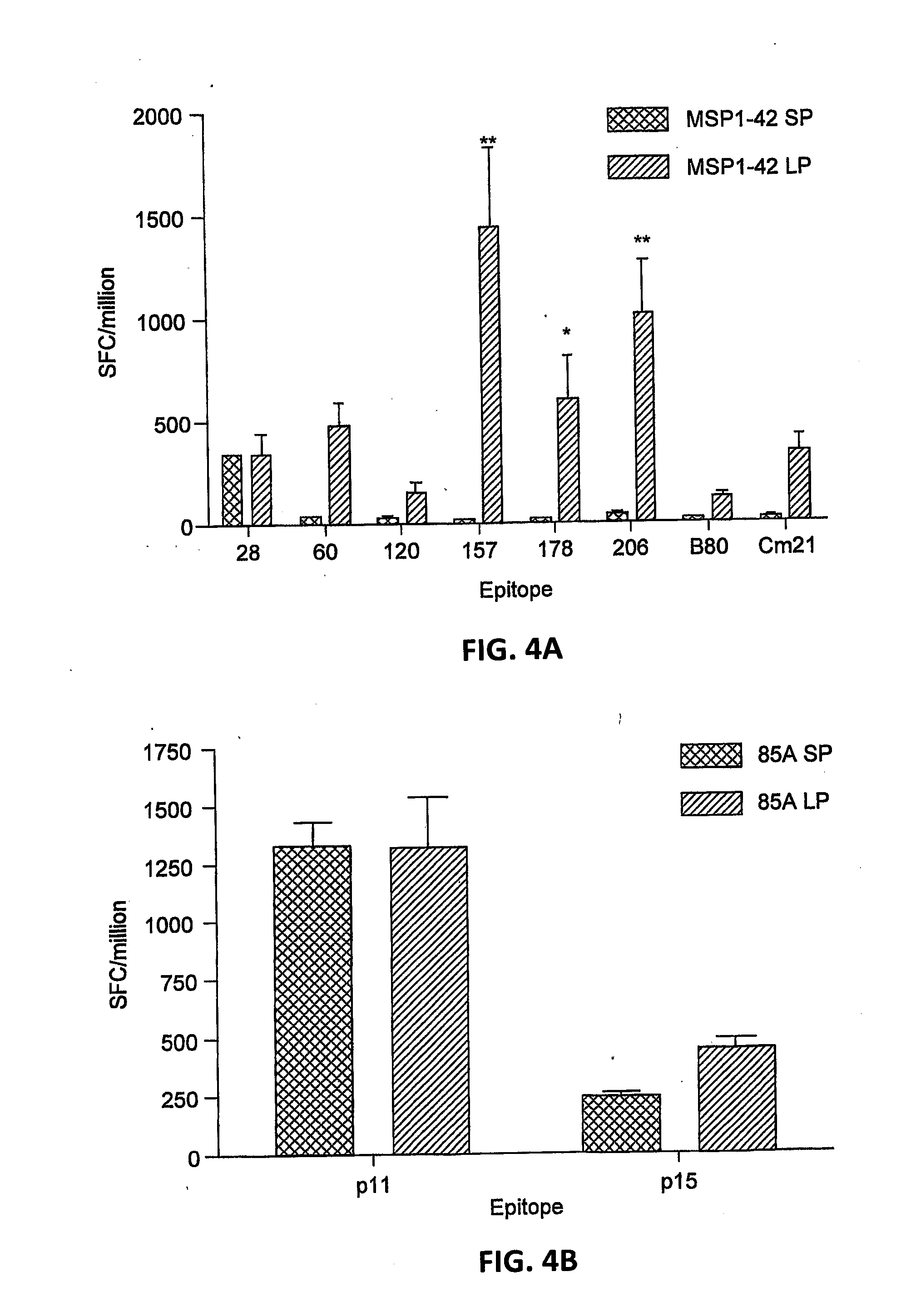
An adenoviral vector comprising a promoter further comprising a fragment of the 5′ untranslated region of the CMV IE1 gene including intron A and a nucleic acid sequence encoding a pathogen or tumour antigen for use as a medicament.
Owner:OXFORD UNIV INNOVATION LTD
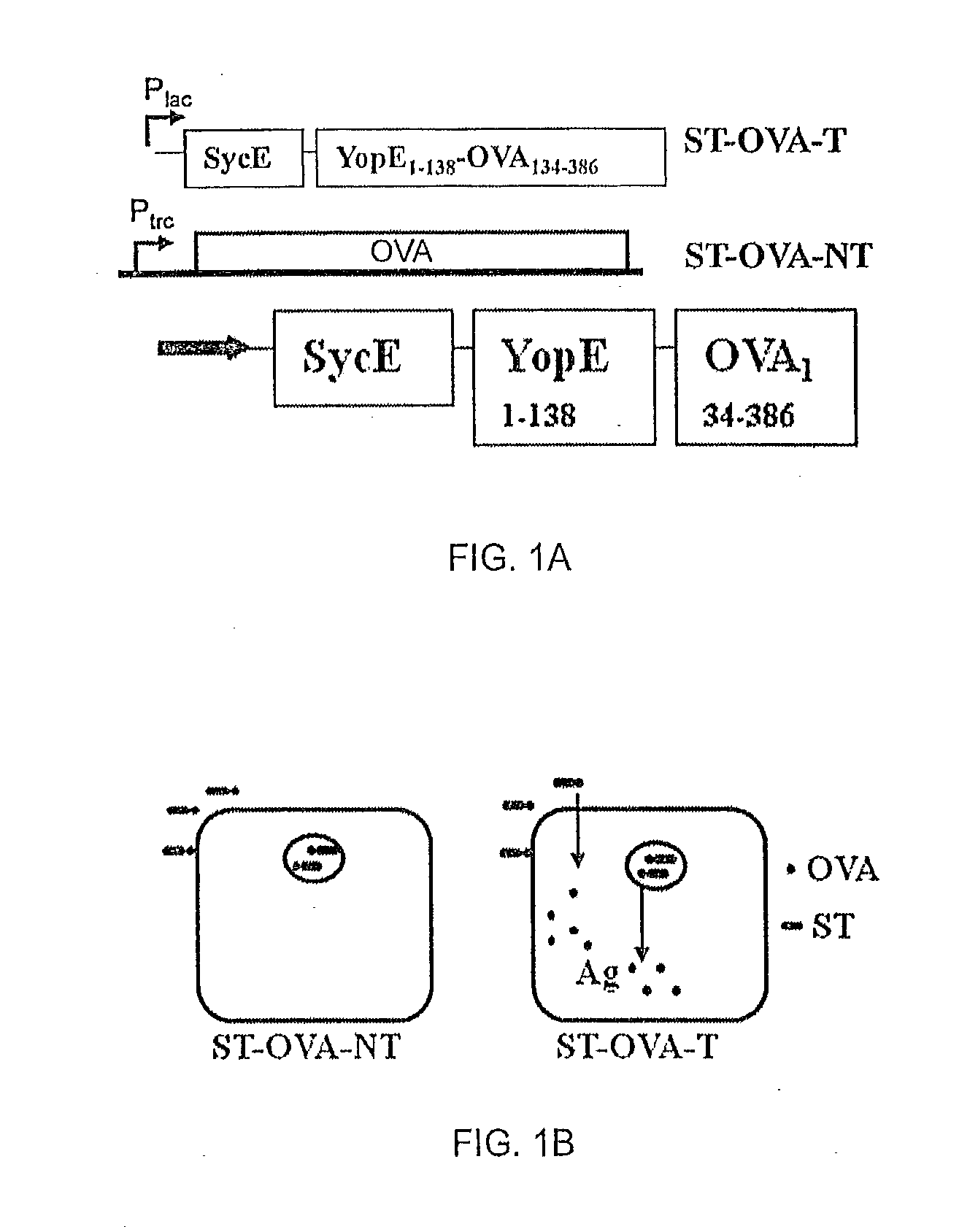
Recombinant Bacterium and Uses Thereof
InactiveUS20130156809A1Effective controlSafe and cost-effectiveSsRNA viruses negative-senseAntibacterial agentsBacteroidesType three secretion system
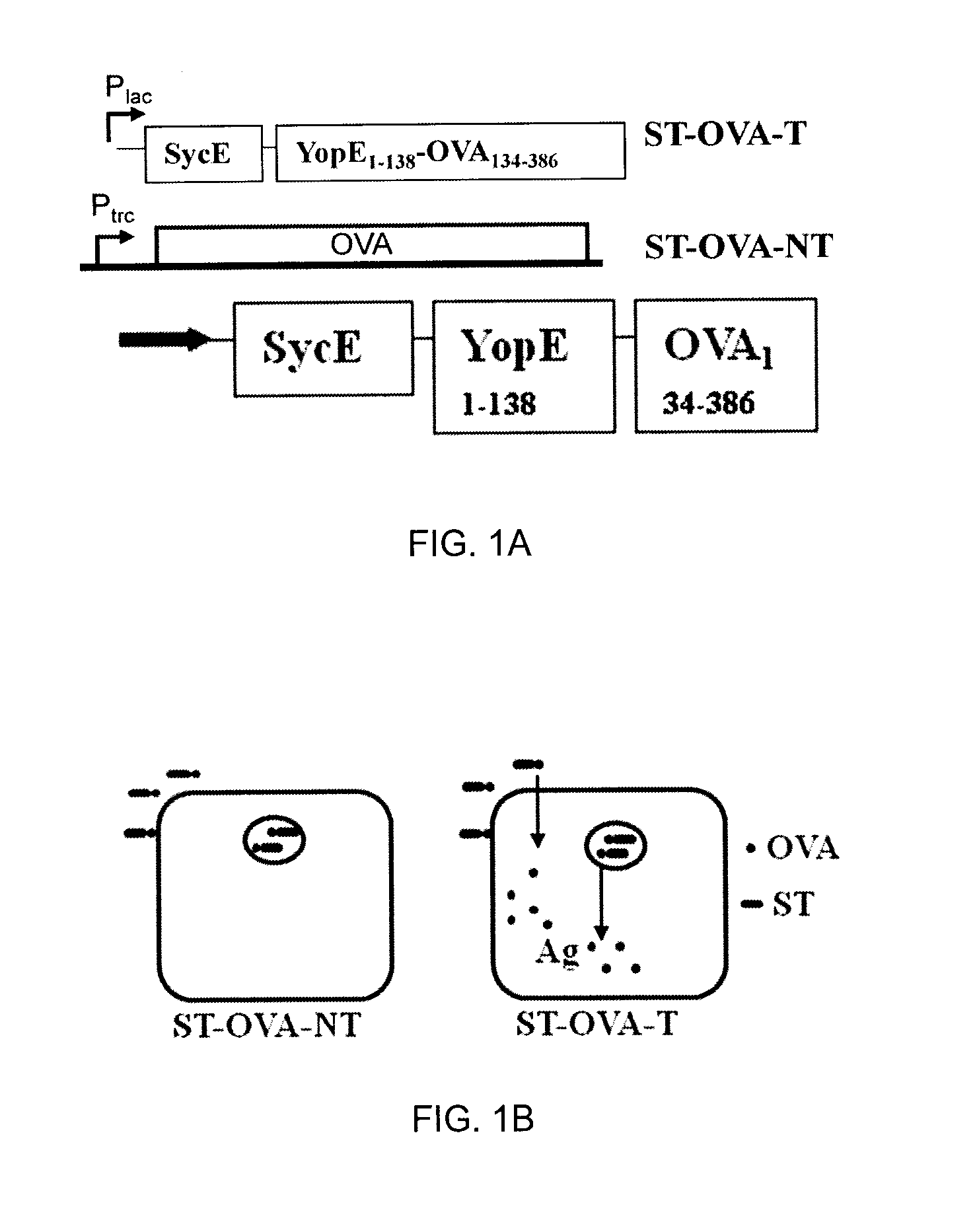
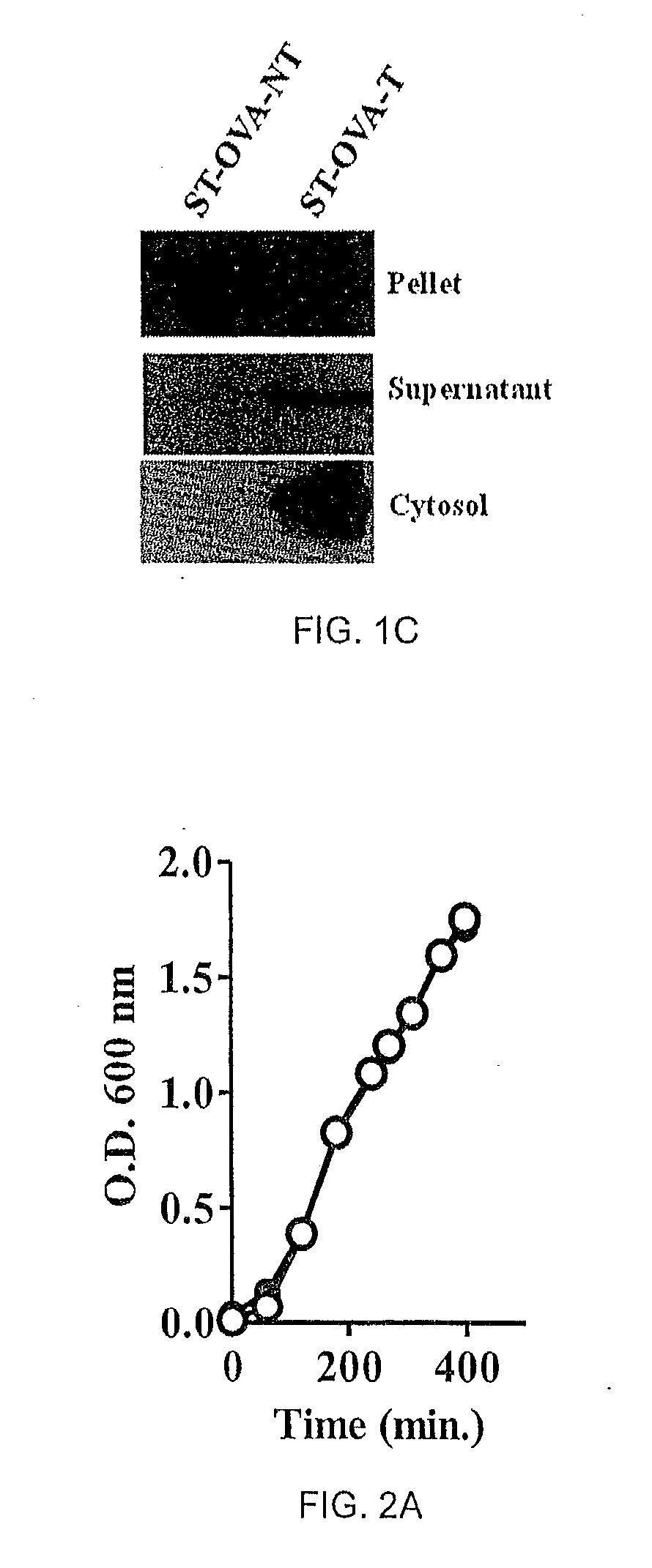
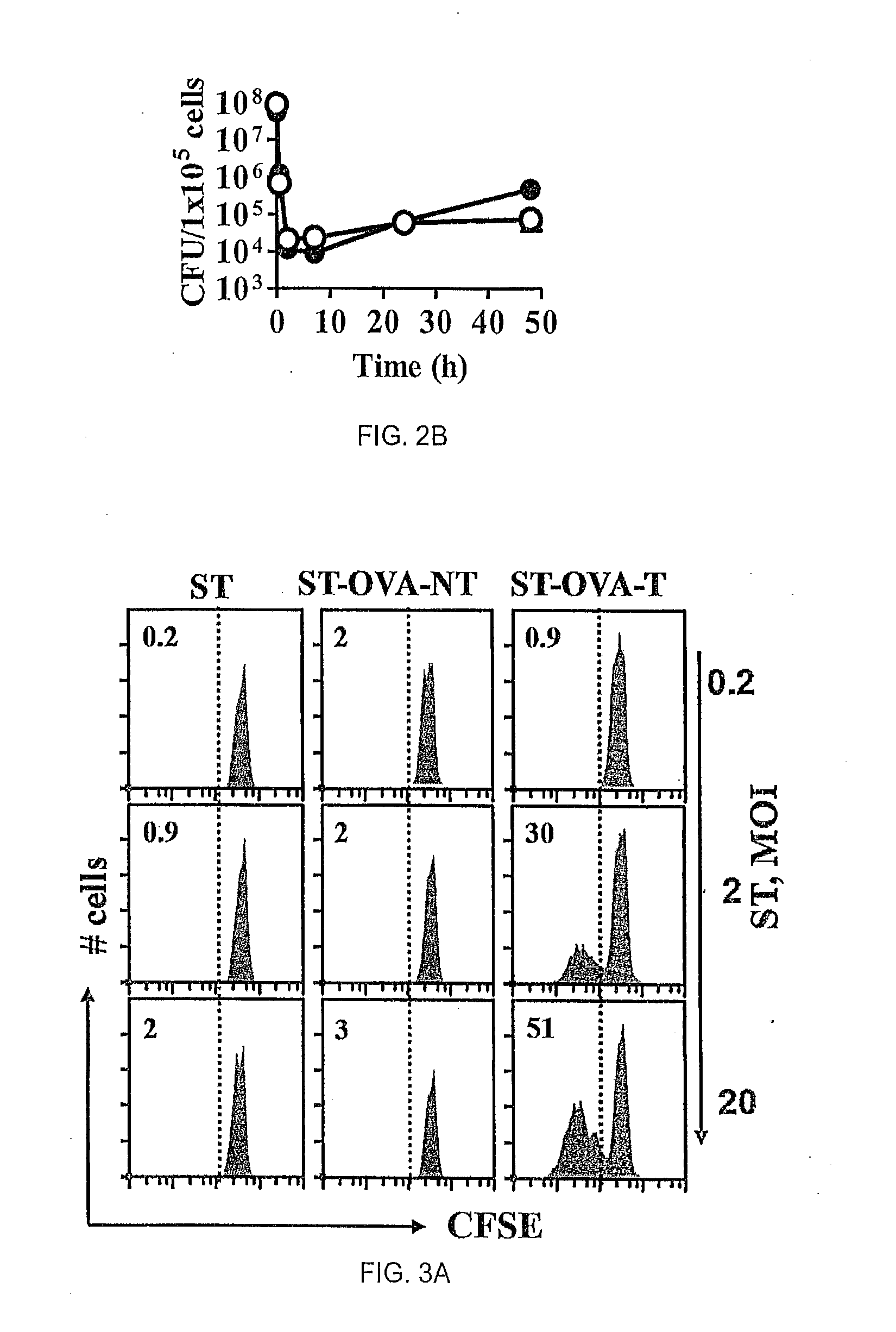
The present invention relates to a recombinant bacterium expressing an antigen that is translocated to the cytosol of a host organism, and uses thereof. To this end, the present invention provides a recombinant bacterium comprising a nucleic acid encoding an antigen that is translocated to the cytosol of a host cell utilizing Type III secretion system. The recombinant bacterium is generally chosen from intracellular pathogens that reside in the phagosome and fail to induce rapid T cell activation. The translocated antigen may be a viral antigen, a bacterial antigen, or a tumour antigen. Methods of imparting immunity using the recombinant bacterium are also provided.
Owner:NAT RES COUNCIL OF CANADA
Antigen composition, preparation method and application of antigen composition and tumour vaccine
InactiveCN103372203AMammal material medical ingredientsCancer antigen ingredientsDendritic cellStage melanoma
The invention provides a preparation method of an antigen composition. The preparation method of the antigen composition comprises the following steps of: (1) mixing soluble protein of melanoma cells with soluble protein of lung adenocarcinoma cells to obtain tumour antigen protein, or mixing melanoma cells with lung adenocarcinoma cells and extracting soluble protein, so as to obtain tumour antigen protein; (2) contacting the tumour antigen protein with immature dendritic cells; (3) inducing the immature dendritic cells contacted with the tumour antigen into mature dendritic cells; and (4) separating cell vesidcles of the mature dendritic cells. The invention also provides antigen composition obtained by adopting the preparation method of the antigen composition and an application of the antigen composition in preparation of a tumour vaccine. Besides, the invention also provides a tumour vaccine which comprises the antigen composition and immunologic adjuvant.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
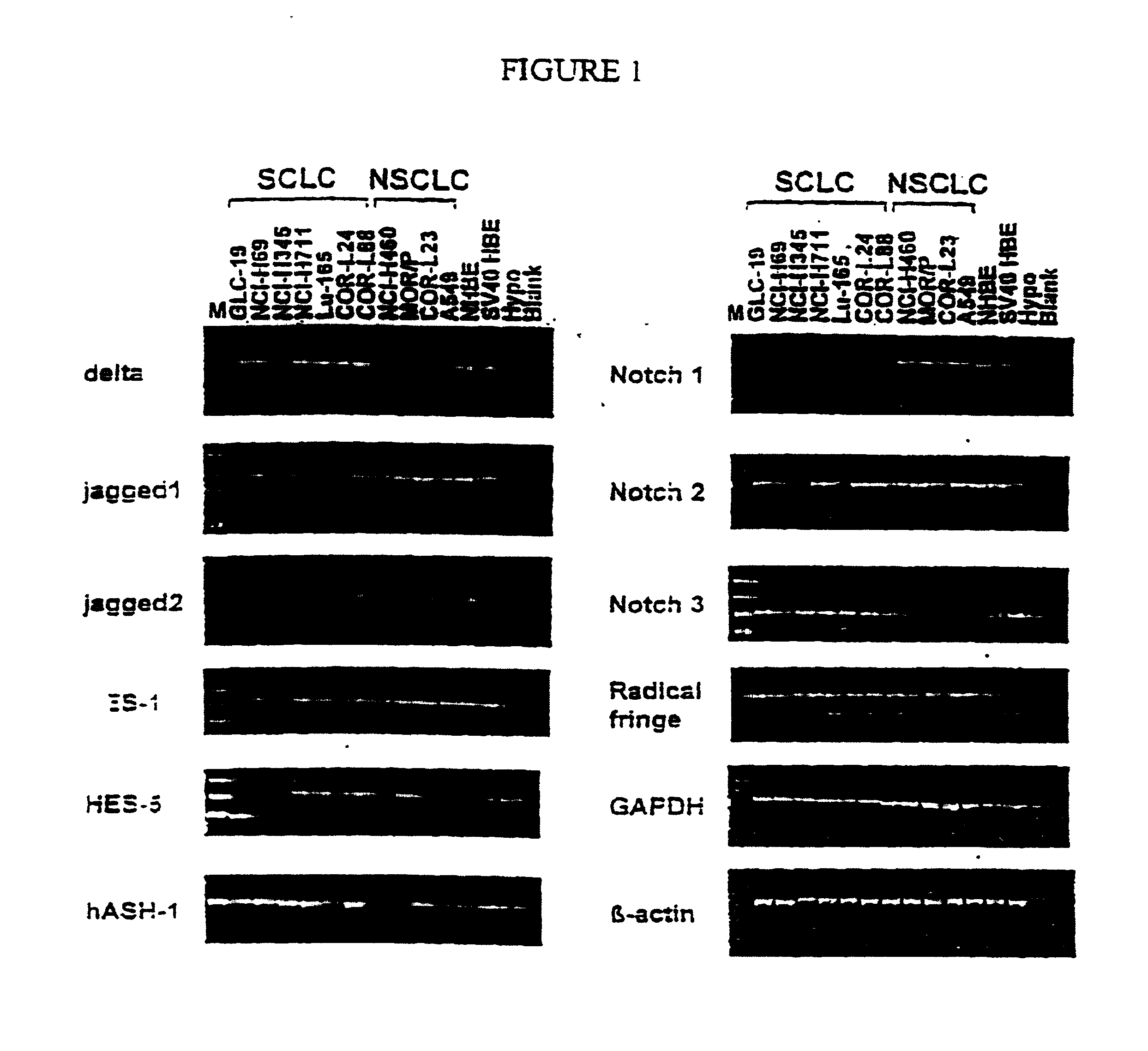
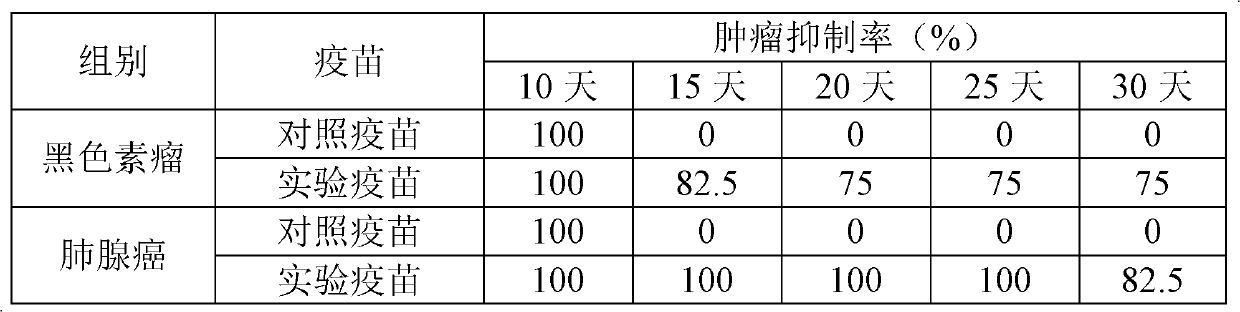
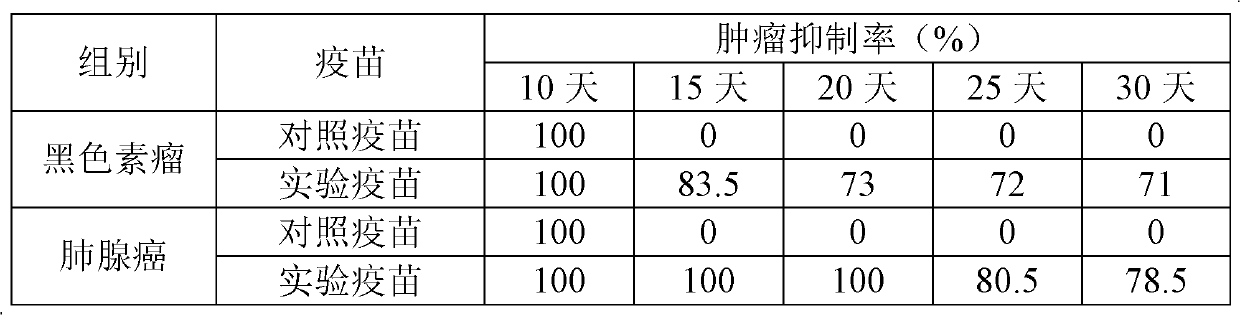
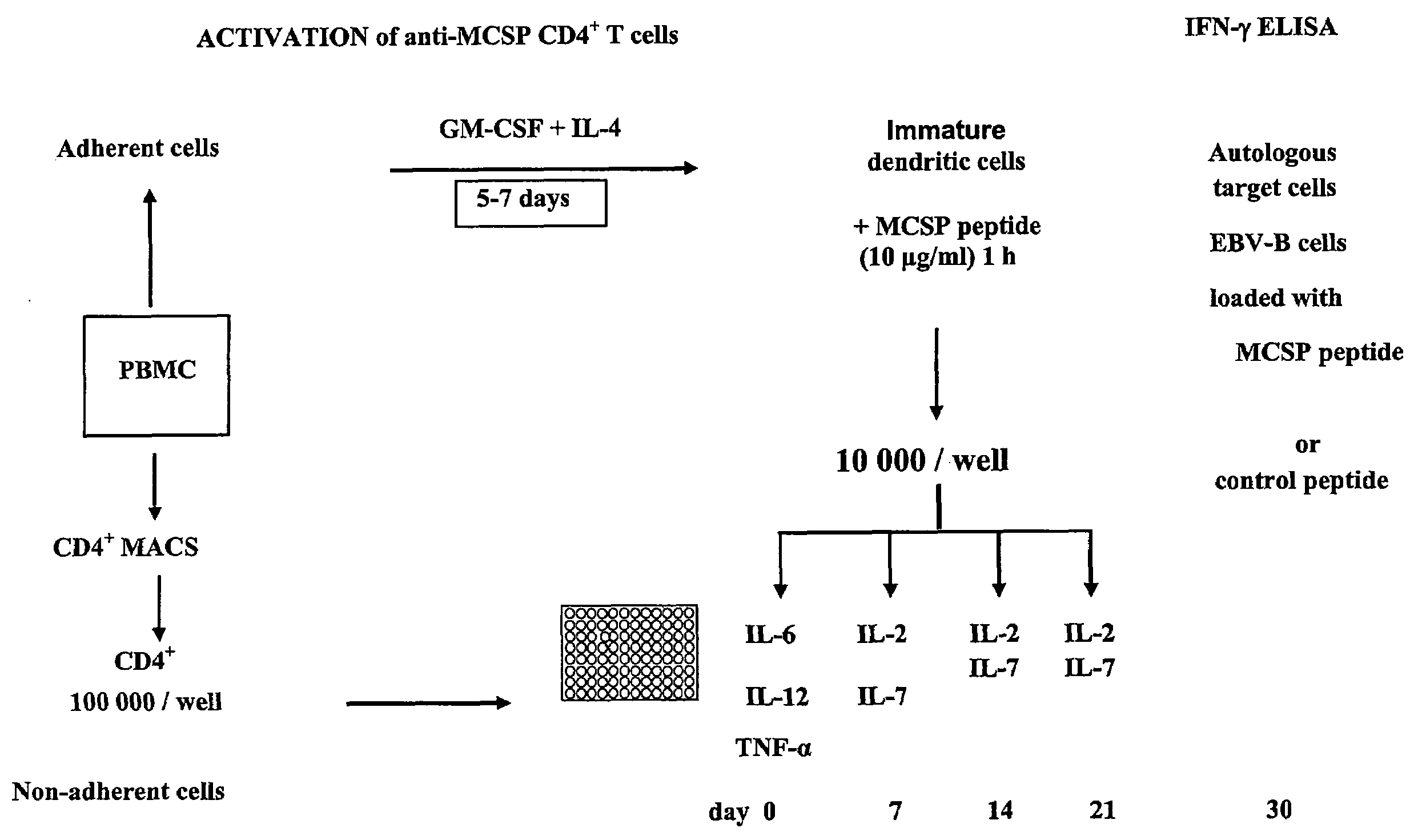
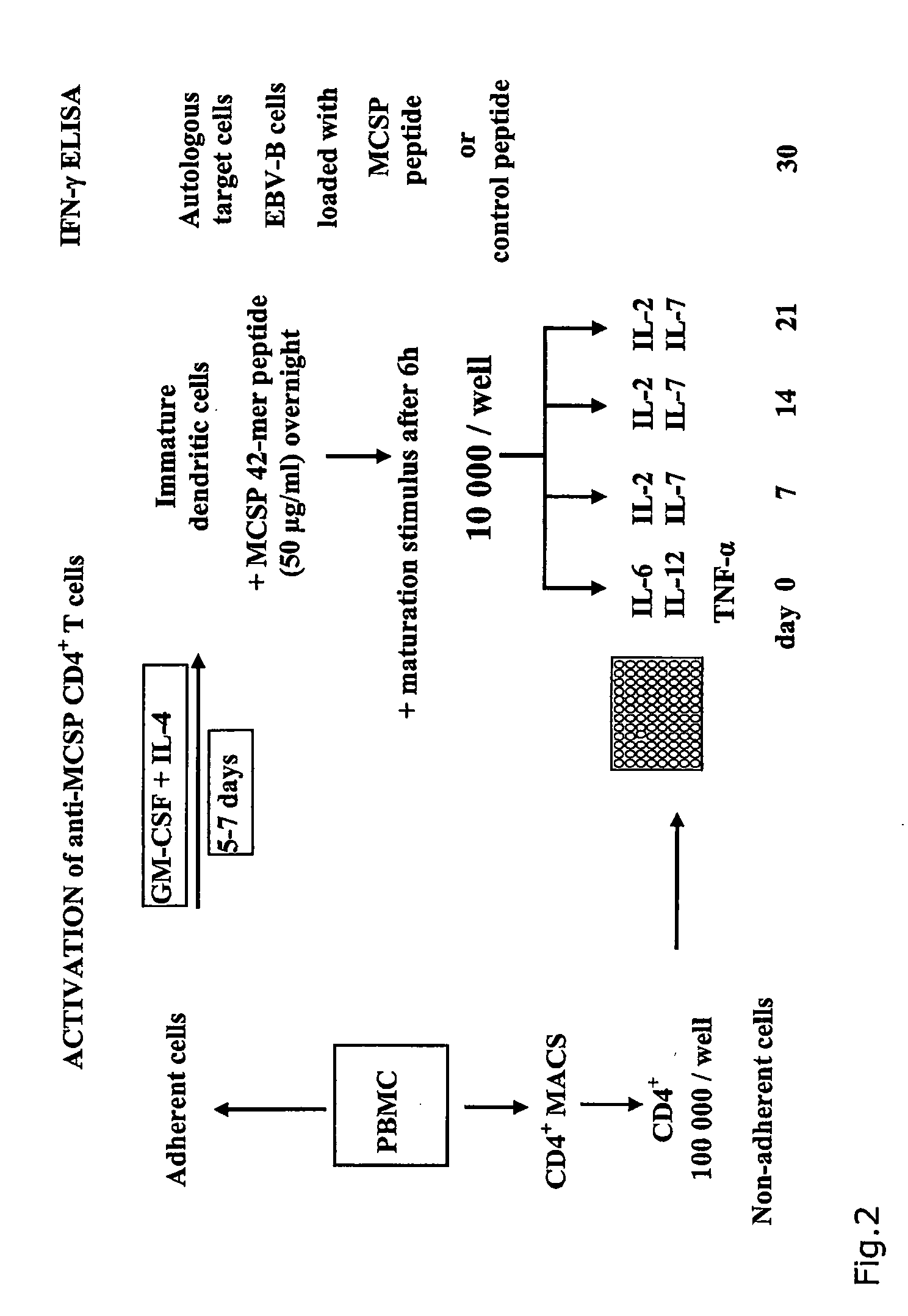
T-Cell Stimulatory Peptides From The Melanoma-Associated Chondroitin Sulfate Proteoglycan And Their Use
The present invention relates to melanoma-associated chondroitin sulfate proteoglycan (MCSP) epitopes recognized by T cells, especially by CD4+ T lymphocytes (short T-cells) and CD8+ T cells, on human melanoma cells. In more detail, the present invention relates to novel T-cell stimulatory tumour antigenic peptides corresponding to said epitopes (MCSP peptides); to fusion proteins comprising said MCSP peptides; to the use of said MCSP peptides, fusion proteins or of the full length MCSP protein itself or fragments thereof to induce an immune response, especially a T-cell response; to the use of said MCSP peptides, fusion proteins or full length MCSP protein itself or fragments thereof to prepare immune cells, such as mature dendritic cells (DCs) loaded with anyone of the peptides according to the invention, or peptide-specific T-cell clones, especially CD4+ or CD8+ T cell clones; to the use of said MCSP peptides, fusion proteins or MCSP itself or fragments thereof for research and development on / of a cancer treatment; to the use of said MCSP peptides, fusion proteins or MCSP itself or fragments thereof for preparing a medicament for inducing a T cell response in a patient, preferably for the treatment of cancer, more preferably for the treatment of melanoma, including cutaneous and ocular melanoma, and other MCSP expressing tumours such as breast cancer, notably lobular breast carcinoma, astrocytoma, glioma, glioblastoma, neuroblastoma, sarcoma and certain types of leukaemia; to the use of said MCSP peptides, fusion proteins or full length MCSP protein or fragments thereof for the preparation of a medicament, and a diagnostic agent for the treatment and prophylaxis as well the diagnosis of an immune response against tumours; and to the use of said peptide-specific T-cell clones for diagnosing or treating cancer.
Owner:SCHULTZ ERWIN +1
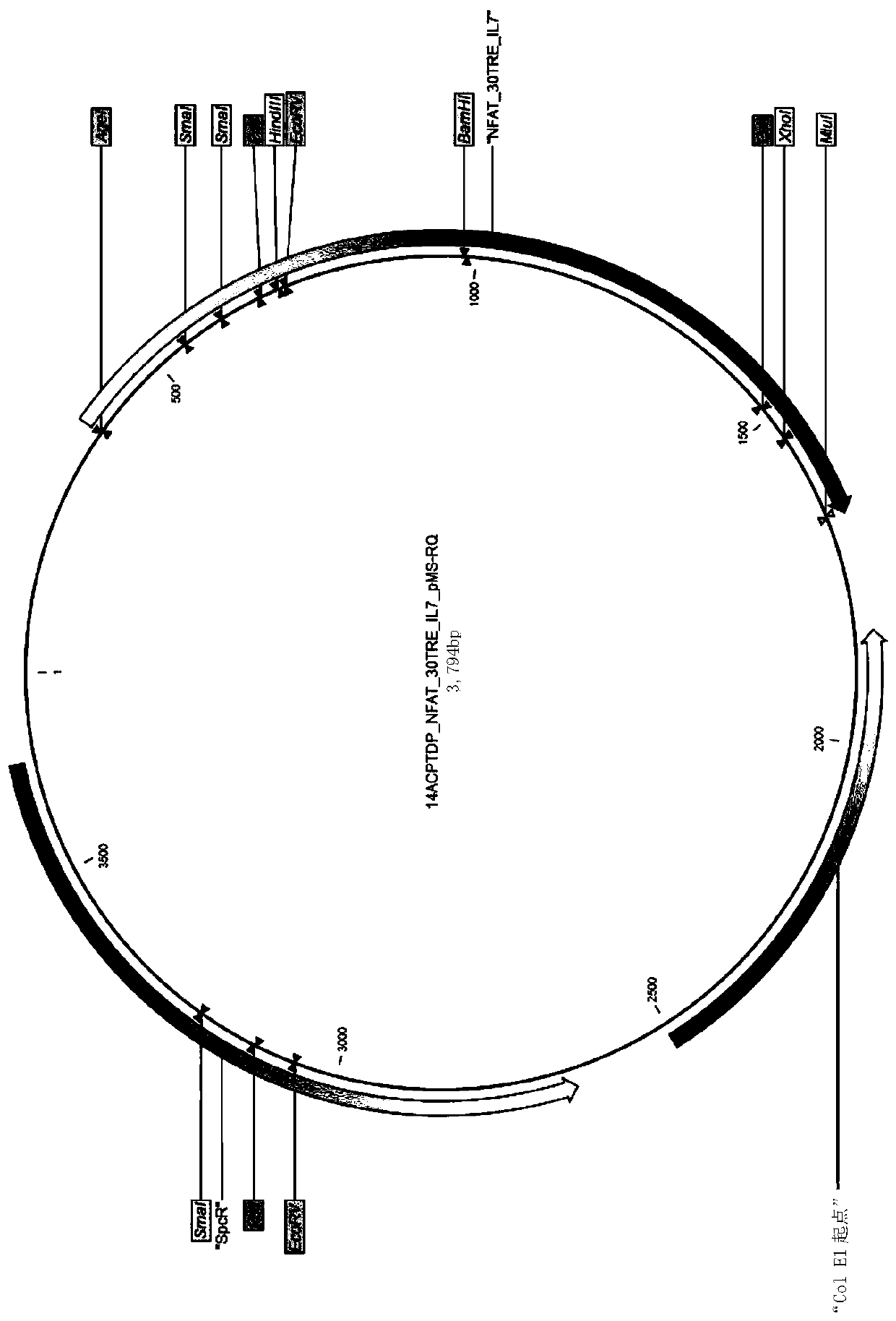
T cell modification
PendingCN111315402APolypeptide with localisation/targeting motifImmunoglobulin superfamilyNucleotideNucleic acid
This invention relates to modified T cells that inducibly express a bioactive molecule, such as IL-7, and constitutively expresses an antigen receptor, such as a T cell receptor or chimeric antigen receptor that binds to a tumour antigen. The modified T cells may comprise a nucleic acid construct that comprises a first nucleotide sequence encoding the bioactive molecule,a second nucleotide sequence encoding the antigen receptor;an inducible promoter operably linked to the first nucleotide sequence and a constitutive promoter operably linked to the second nucleotide. Nucleic acid constructs andvectors are provided, as well as T cells comprising such constructs and vectors and therapeutic methods and uses thereof.
Owner:ADAPTIMMUNE
Recombinant Bacterium and Uses Thereof
ActiveUS20150140028A1Effective controlQuick responseAntibacterial agentsSsRNA viruses negative-senseBacteroidesType three secretion system
The present invention relates to a recombinant bacterium expressing an antigen that is translocated to the cytosol of a host organism, and uses thereof. To this end, the present invention provides a recombinant bacterium comprising a nucleic acid encoding an antigen that is translocated to the cytosol of a host cell utilizing Type III secretion system. The recombinant bacterium is generally chosen from intracellular pathogens that reside in the phagosome and fail to induce rapid T cell activation. The translocated antigen may be a viral antigen, a bacterial antigen, or a tumour antigen. Methods of imparting immunity using the recombinant bacterium are also provided.
Owner:NAT RES COUNCIL OF CANADA
A method to up-regulate cancer stem cell markers for the generation of antigen specific cytotoxic effector T cells
InactiveCN107148470AMammal material medical ingredientsCancer antigen ingredientsDendritic cellReprogramming
Owner:AGENCY FOR SCI TECH & RES
Novel Adenovirus Vectors
ActiveUS20150297700A1Increase the lengthImprove efficacyEnzymologyImmunoglobulinsNucleic acid sequencingPromoter
An adenoviral vector comprising a promoter further comprising a fragment of the 5′ untranslated region of the CMV IE1 gene including intron A and a nucleic acid sequence encoding a pathogen or tumour antigen for use as a medicament.
Owner:OXFORD UNIV INNOVATION LTD
Combination
InactiveUS20170252435A1More accessImprove breathabilityPolypeptide with localisation/targeting motifPeptide/protein ingredientsRGD motifTarget peptide
A pharmaceutical product comprising (a) a conjugation product of TNF and a tumour- or tumour vasculature-targeting peptide comprising an NGR, DGR, isoDGR or RGD motif; and (b) a cell comprising a chimeric antigen receptor (CAR), wherein the CAR comprises an antigen-specific targeting domain which targets a tumour antigen.
Owner:MOLMED SPA
A method to up-regulate cancer stem cell markers for the generation of antigen specific cytotoxic effector t cells
InactiveUS20170191034A1Mammal material medical ingredientsCancer antigen ingredientsDendritic cellSpecific immunity
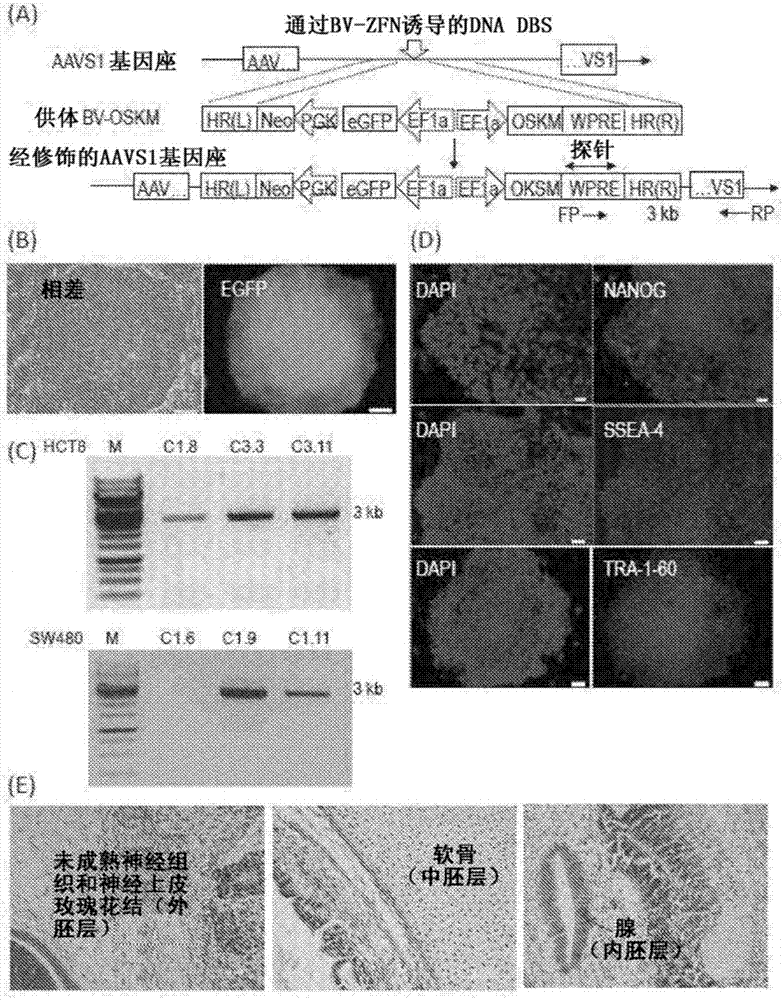
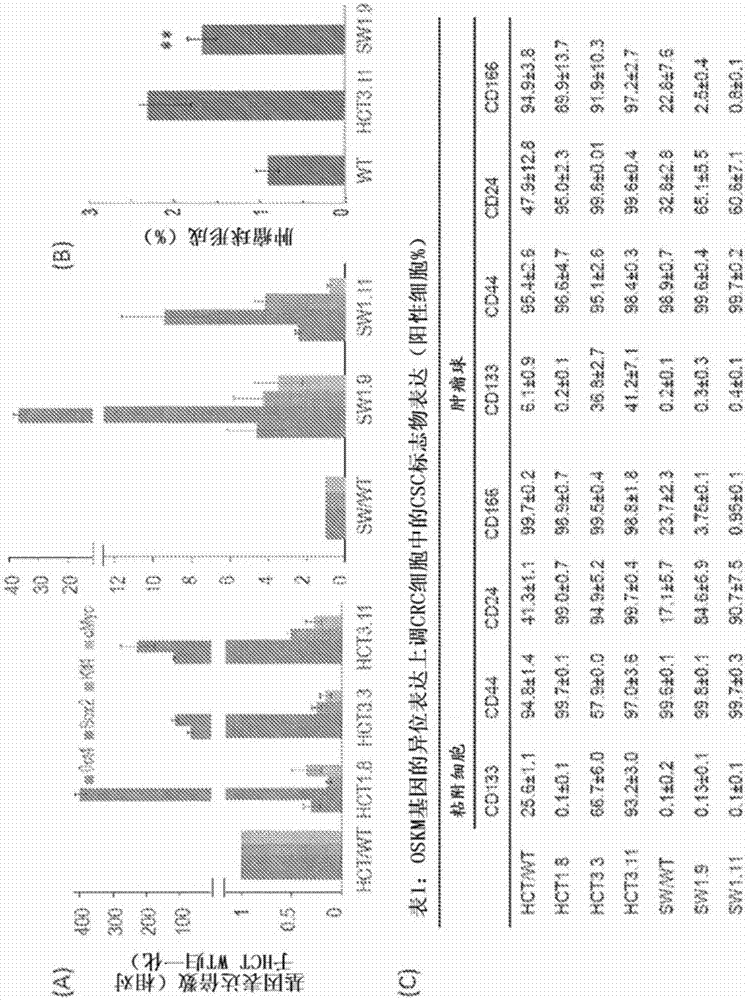
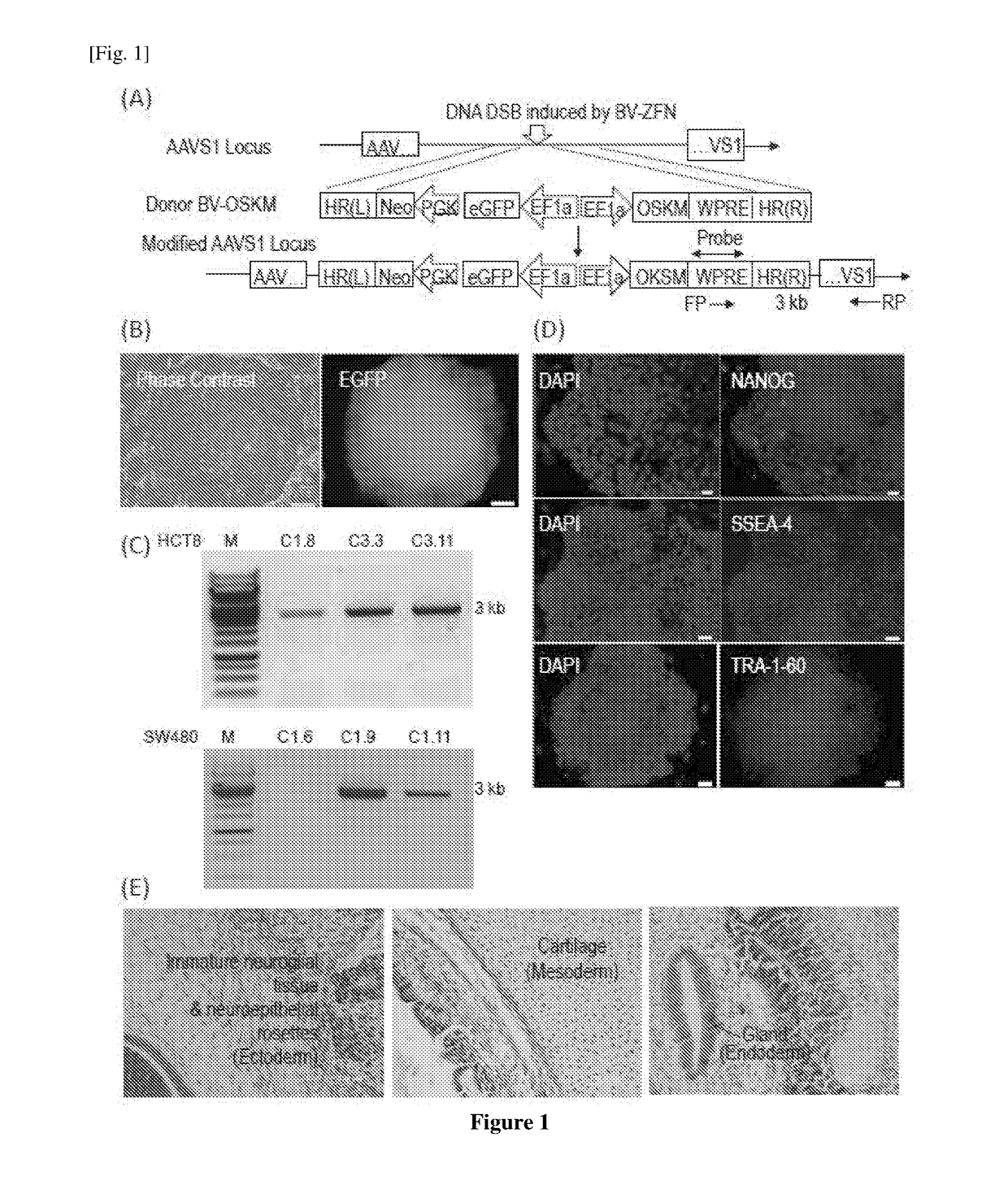
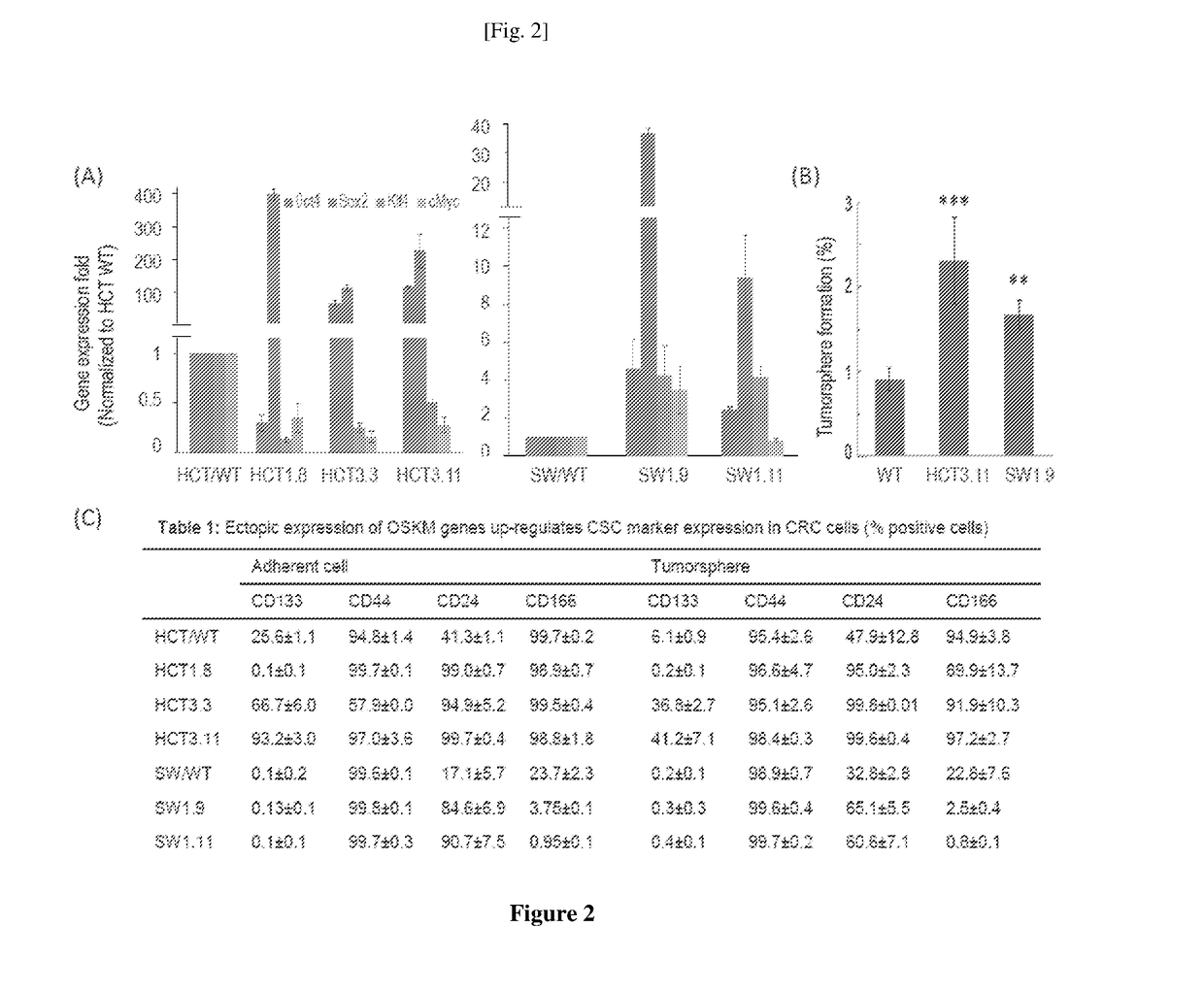
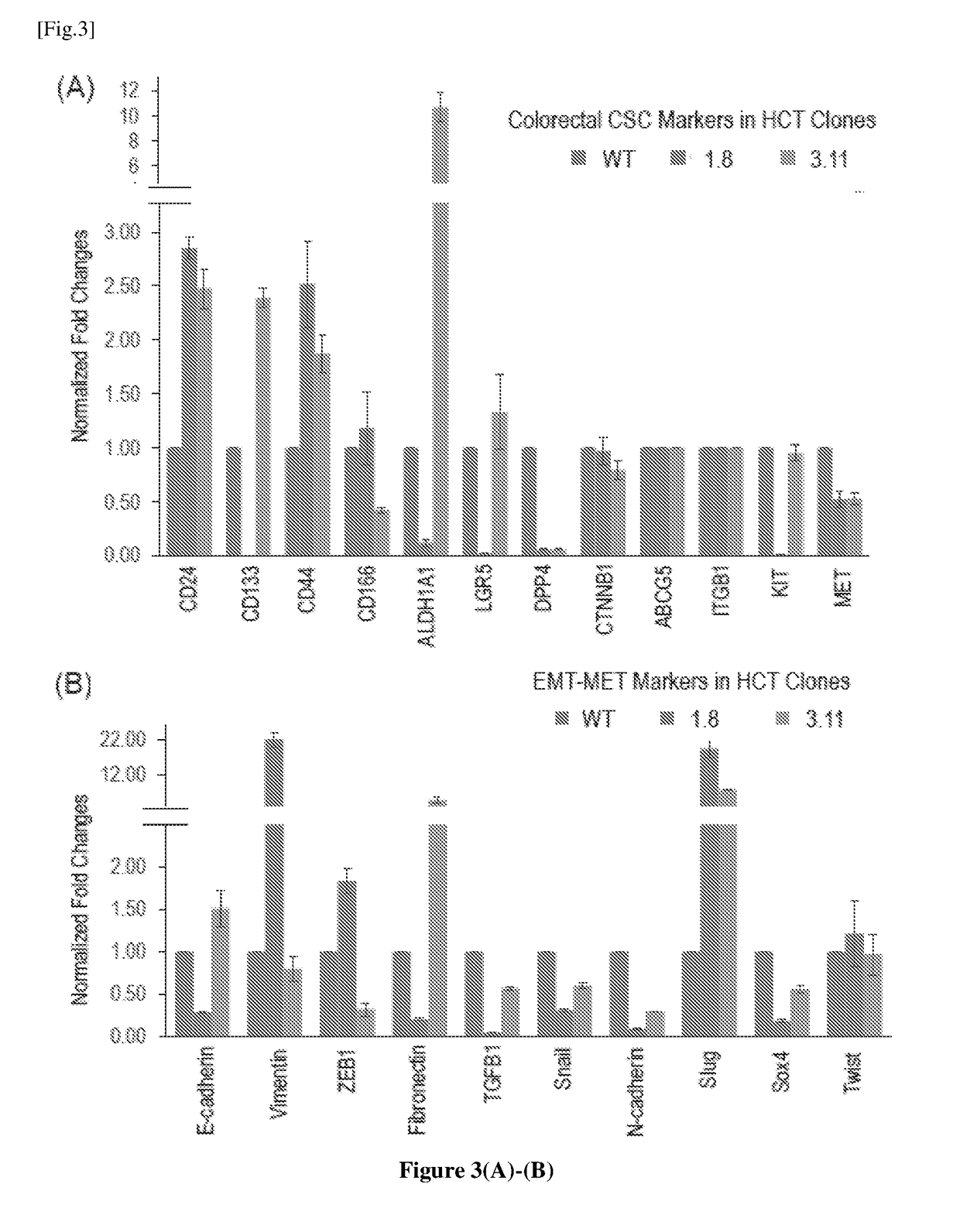
The invention concerns a method of preparing a composition comprising stimulated immune system cells such as dendritic cells (DC) for use in inducing immune response of cytotoxic T lymphocytes against colorectal cancer. The dendritic cells are pulsed by contact with colorectal cancer stem cells (CSC) or their fragments thereof. These colorectal CSCs are produced by OSKM (Oct4, Sox2, Klf4 and c-Myc) induced reprogramming of differentiated colorectal cancer cells (CRC) which results in undifferentiated colorectal CSC-like cells. Both the CSC-like cells and the lysates of heat-shocked CSC-like cells could be used as an accessible source of tumour antigens for DC pulsing to induce specific immune responses against colorectal CSCs.
Owner:AGENCY FOR SCI TECH & RES
Conditional agonists of immune responses
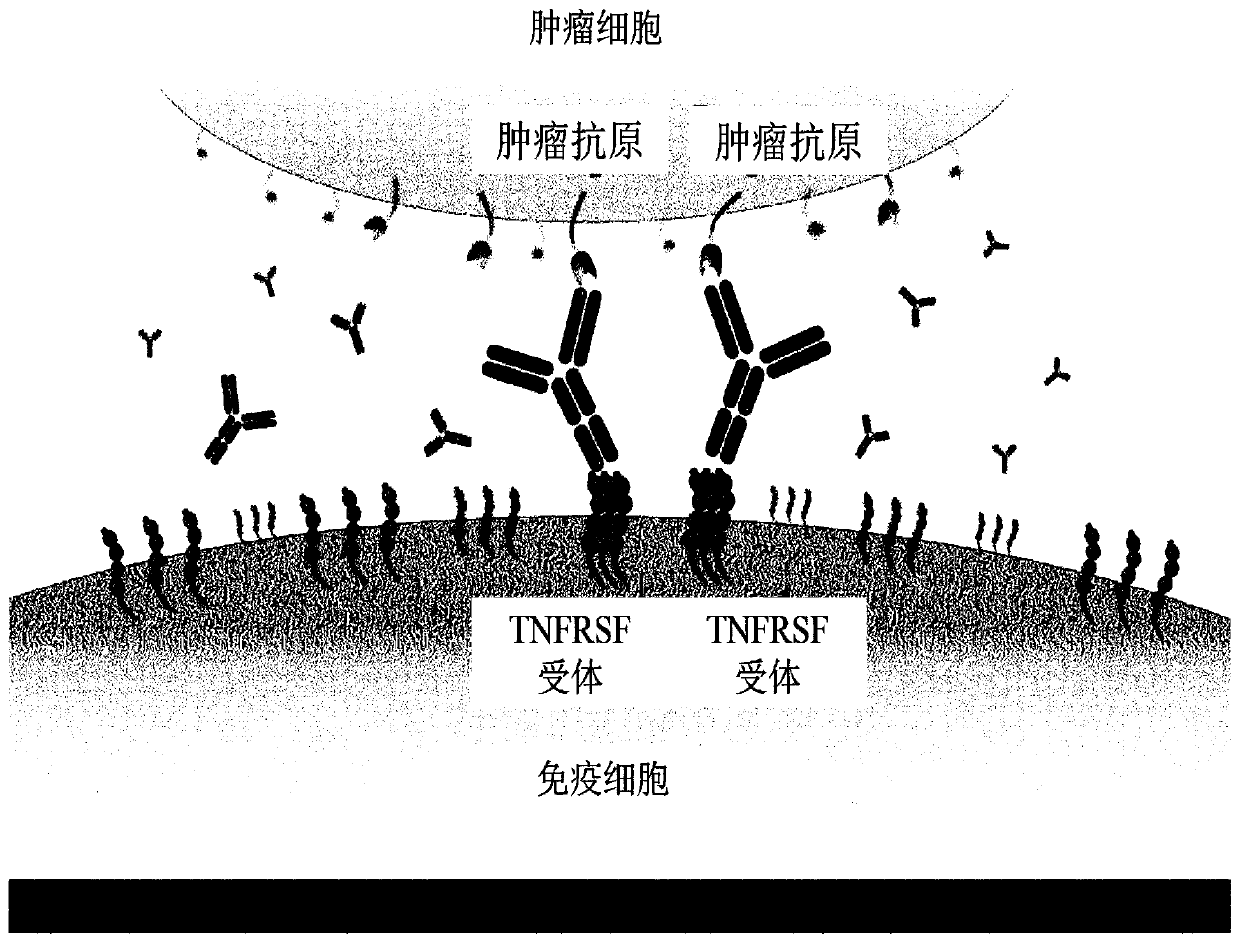
PendingCN110366561ALess clusteringReduce off-target effectsImmunoglobulins against growth factorsImmunoglobulins against cell receptors/antigens/surface-determinantsAgonistTumor antigen
The application relates to antibody molecules, which bind to a first and second antigen, the first antigen being a disease antigen, for example a tumour antigen, and the second antigen being a tumournecrosis factor receptor superfamily (TNFRSF) receptor antigen on the surface of an immune cell. The antibody molecules preferably comprise a CDR-based antigen-binding site and an antigen-binding site, which may be located in two or more structural loops of a constant domain of the antibody molecule. The antibody molecules find application, for example, in cancer therapy.
Owner:F STAR THERAPEUTICS LTD
Chimeric antigen receptor (CAR) targeting bispecific sites and application thereof
PendingCN113493516AHigh transduction efficiencyImprove anti-tumor effectVirusesAntibody mimetics/scaffoldsSingle-Chain AntibodiesGenetic engineering
The invention belongs to the technical field of gene engineering, and particularly relates to a bispecific single-chain antibody, a CAR structure containing the bispecific single-chain antibody, an expression vector, an immune cell and application. In the single-chain antibody, the amino acid sequence of a Linker1 structure is shown as SEQ ID NO:17 or a functional variant thereof; the amino acid sequence of a Linker2 structure is shown as SEQ ID NO:18 or a functional variant thereof; the Linker1 is connected with a heavy chain variable region of ScFv1 and a light chain variable region of ScFv2 or is connected with a heavy chain variable region of the ScFv2 and a light chain variable region of the ScFv1; and the Linker2 is connected with the light chain variable region of the ScFv2 and the heavy chain variable region of the ScFv2. The construction of the CAR structure overcomes the technical effect defect of a double-target CAR in the prior art; two different tumour antigens can be targeted at the same time; the killing rate of tumour cells is improved; and the immune escape probability of the tumour cells is reduced.
Owner:CHONGQING PRECISION BIOTECH CO LTD
Tumour antigen protein and tumour antigen peptide
A tumor antigen protein and its tumor antigen peptide used to prepare the medicine for treating lung cancer are disclosed. Said tumor antigen protein can generate the peptide fragment by cell endolysis. Said peptide fragment can bind with the (MHC)-I molecular compatible with main tissue and can be recognized by T cells. The DNA sequence coding said tumor antigen protein has the nucleotide sequence No.AF497803 in GenBank.
Owner:PEKING UNIV
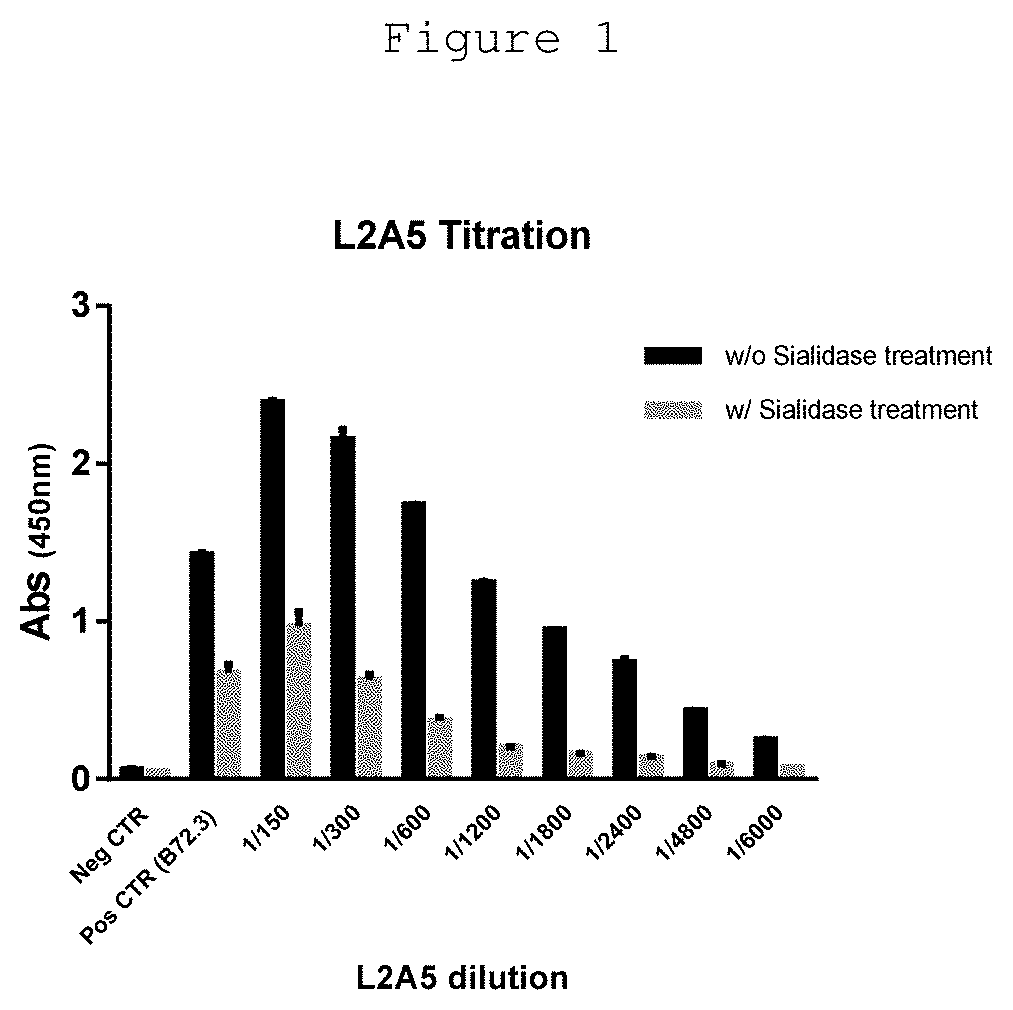
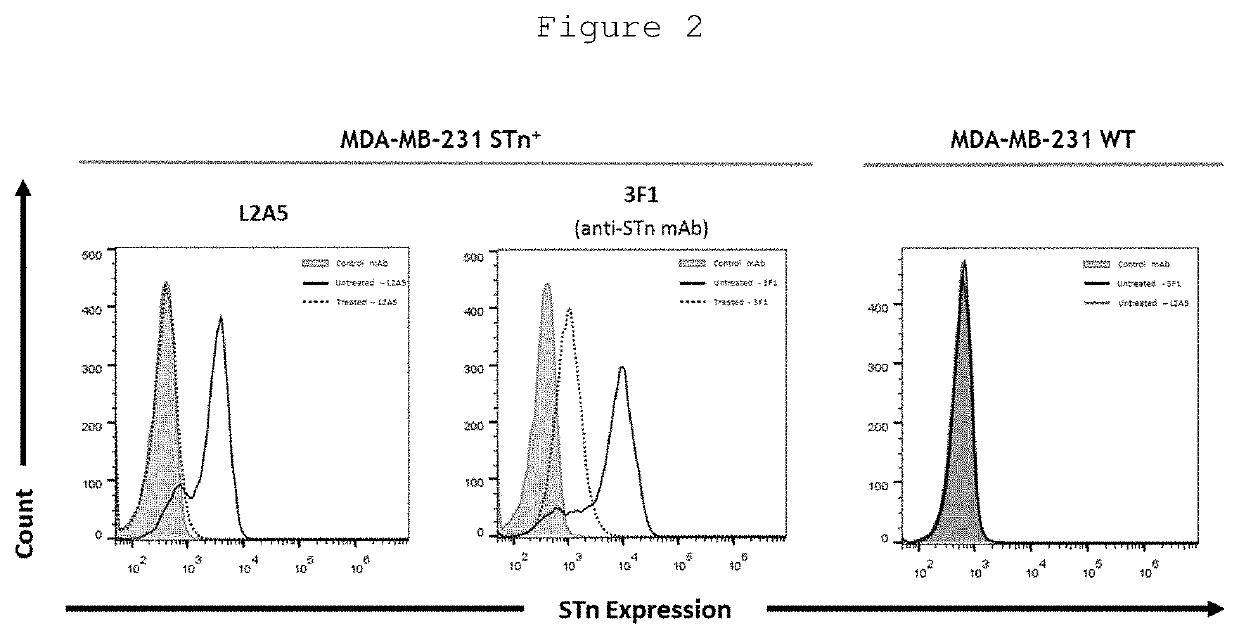
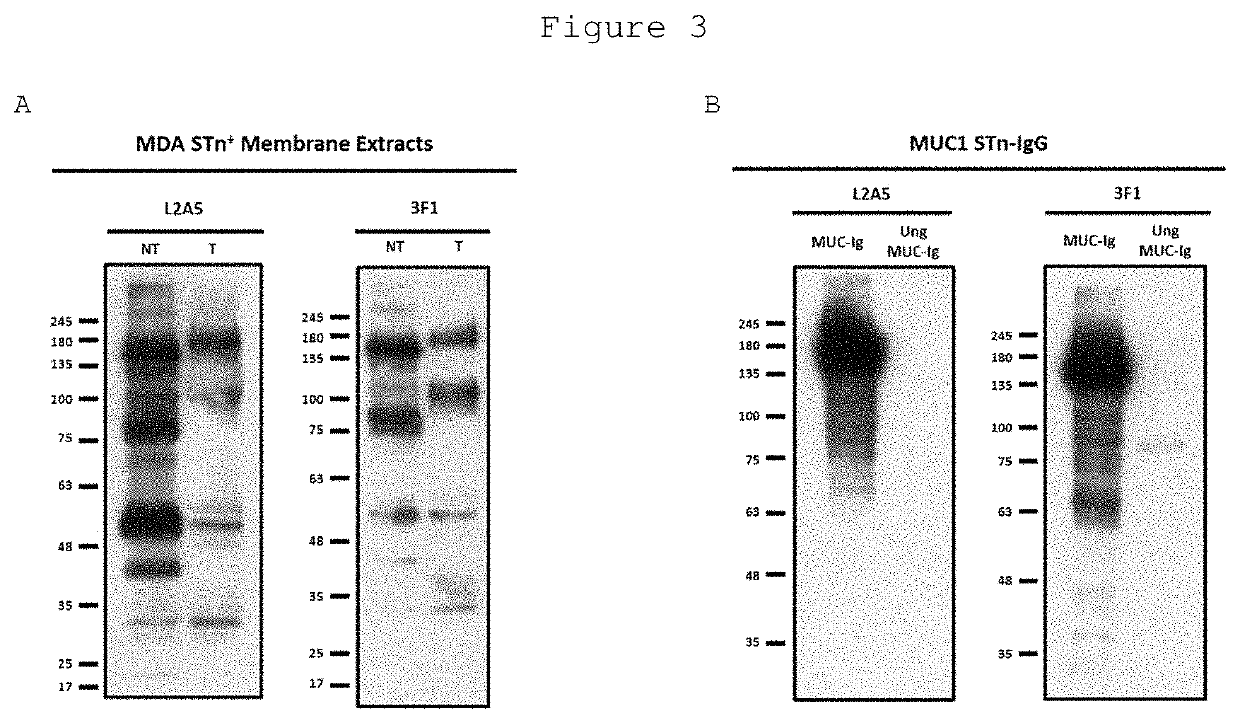
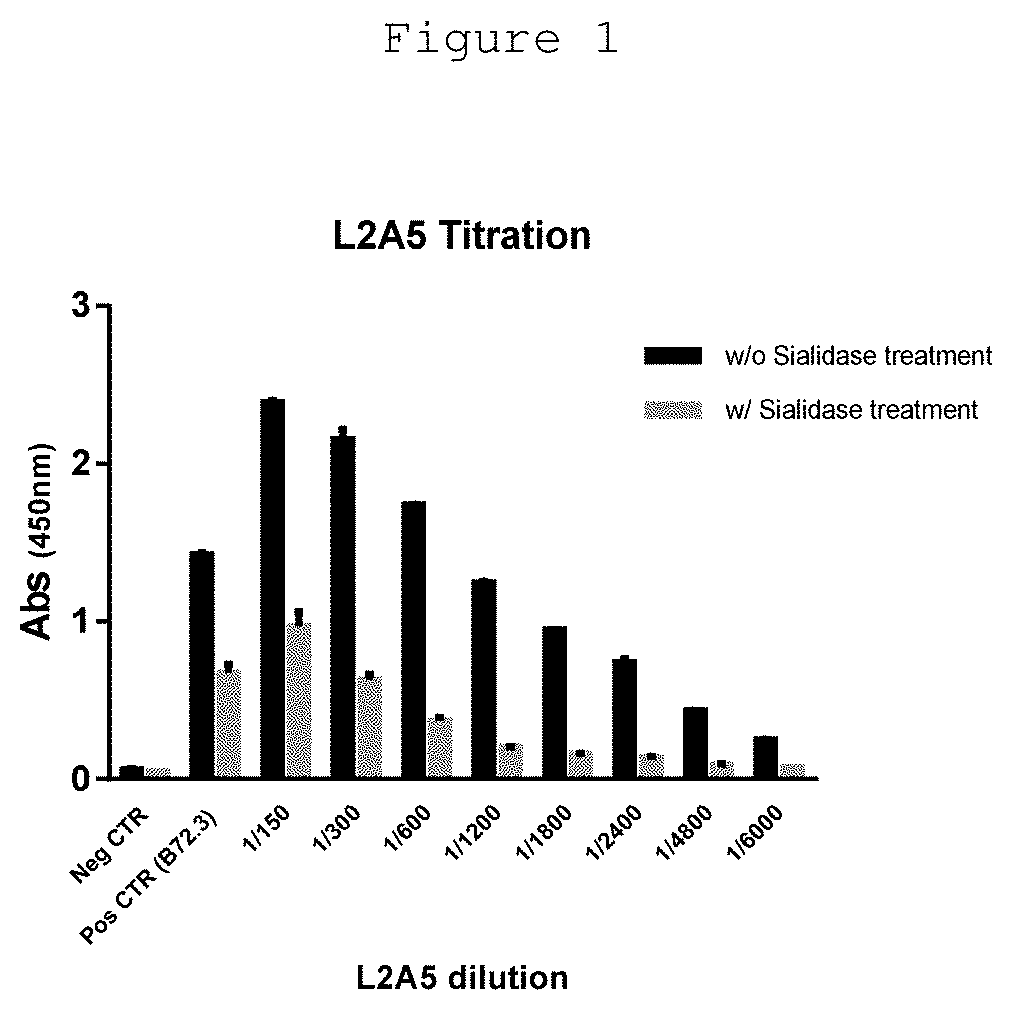
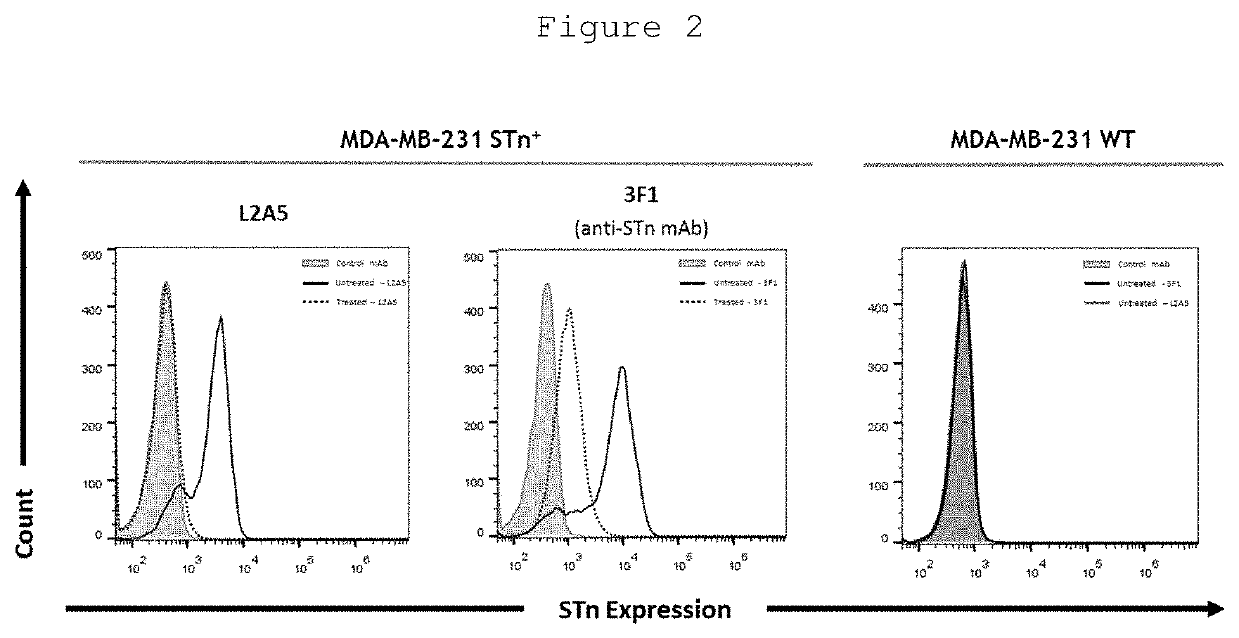
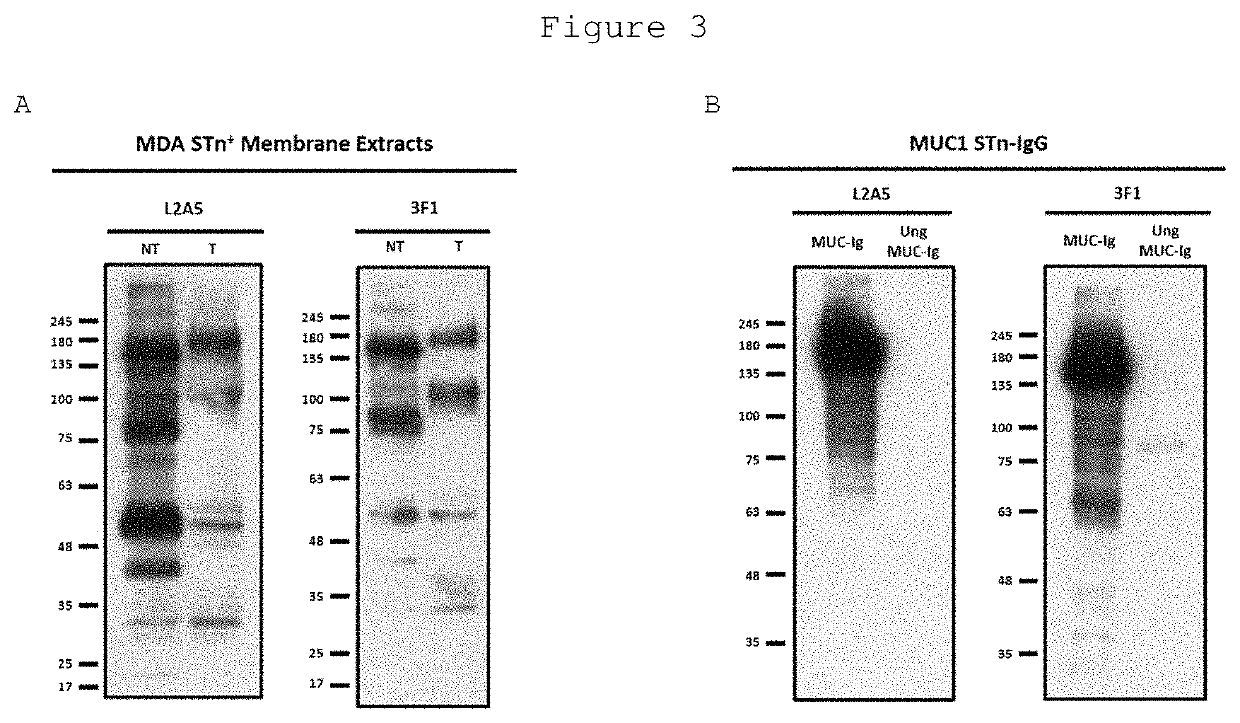
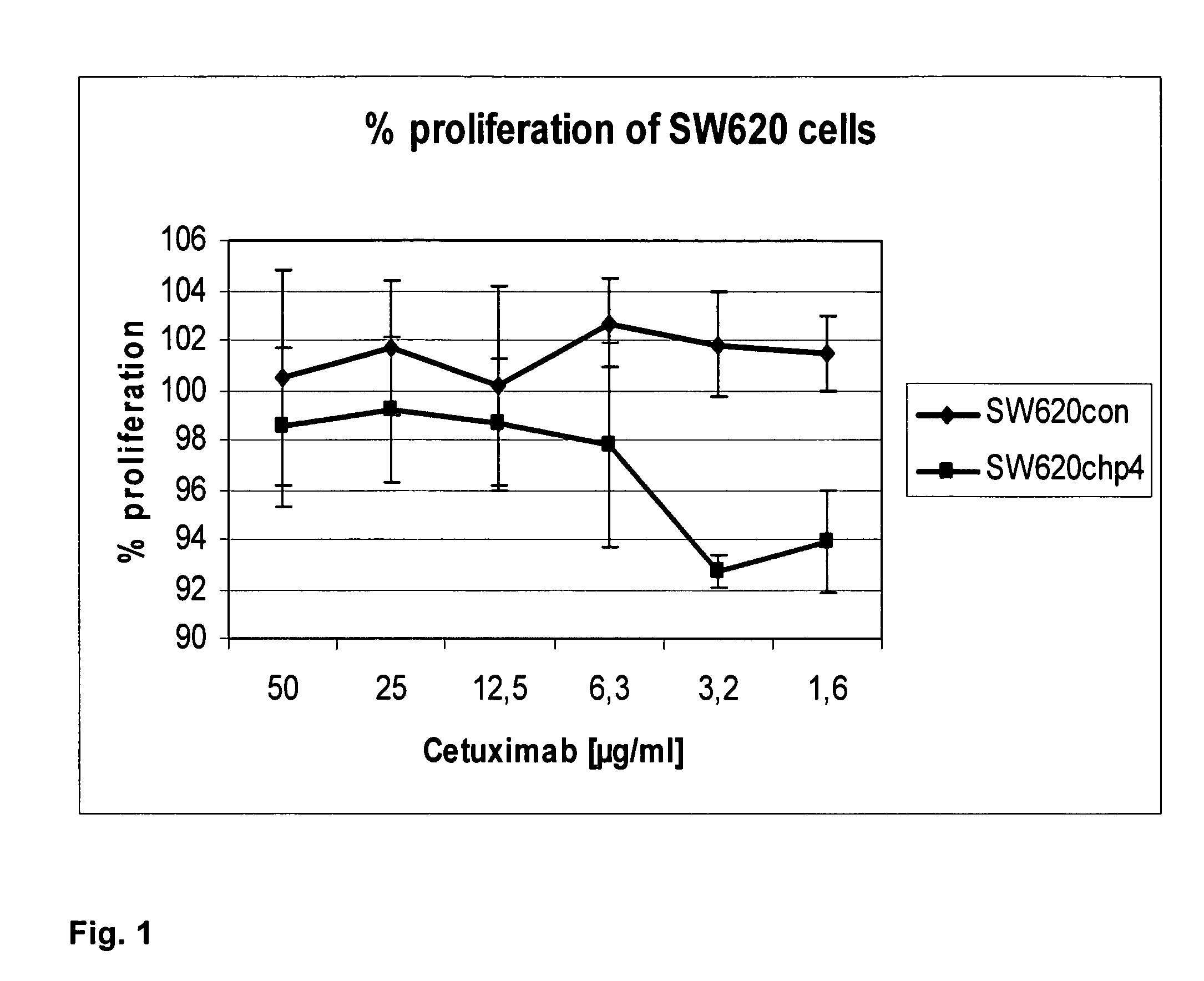
L2a5 antibody or functional fragment thereof against tumour antigens
ActiveUS20210033616A1High extensionIncrease intensityPolypeptide with localisation/targeting motifMicroorganismsAntibody fragmentsNucleotide
This invention provides an antibody or functional antibody fragments, or probe thereof directed against a unique group of antigens identified in cancer.The present invention comprises nucleotide sequences derived from L2A5 monoclonal antibody. The antibody or functional antibody fragment, or probe thereof includes a variable heavy chain domain and a variable light chain domain that has an amino acid sequence provided herein. This DNA / amino acid sequence conjugation is unique and has never been described before. The present invention further provides antibody or functional antibody fragment or a conjugate or a recombinant protein useful in the detection, treatment and prevention of human disease, including cancer.
Owner:UNIV NOVA DE LISBOA +2
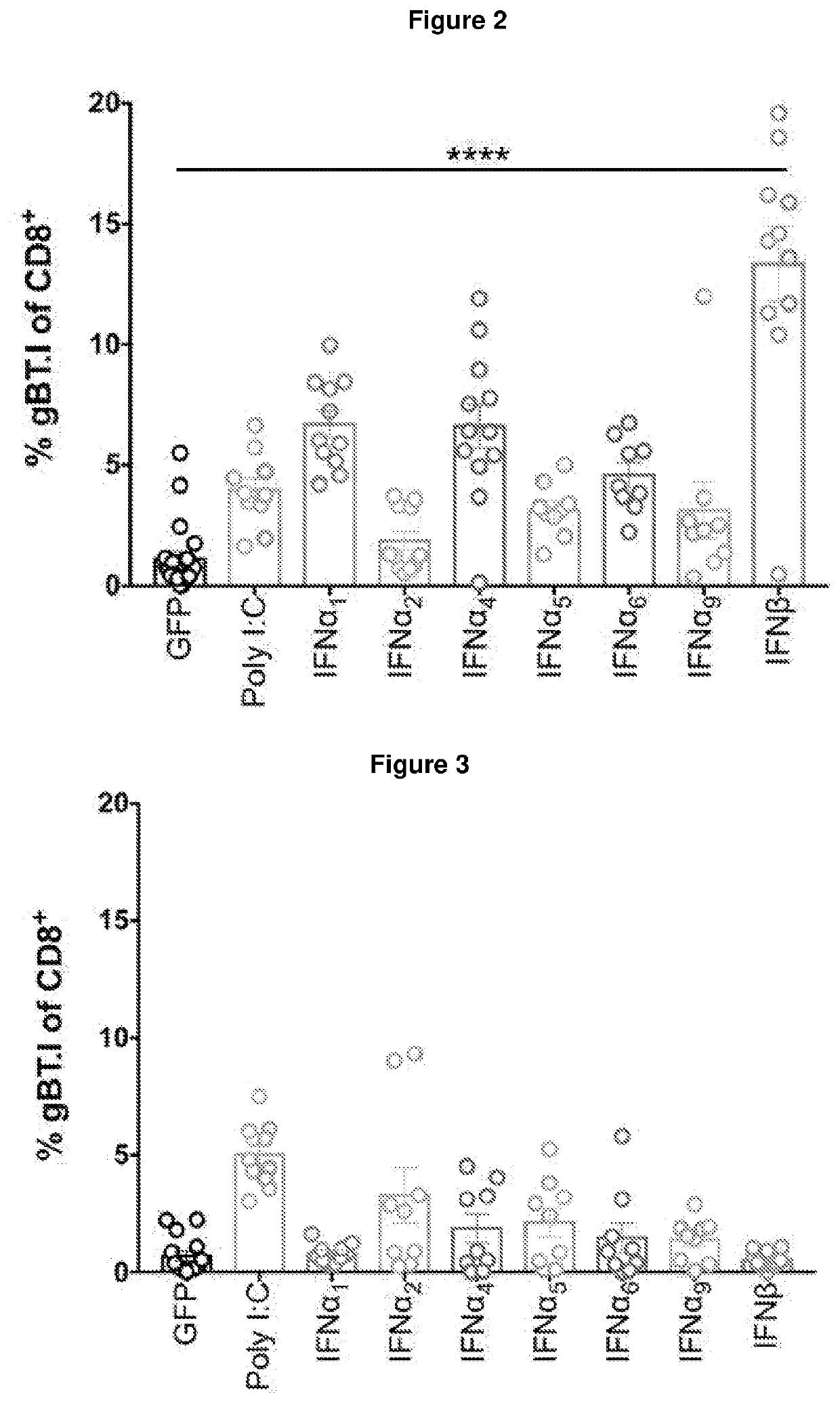
Immunogenic compositions
PendingUS20220226449A1Reduce riskPeptide/protein ingredientsAntibody ingredientsAgonistTreatment use
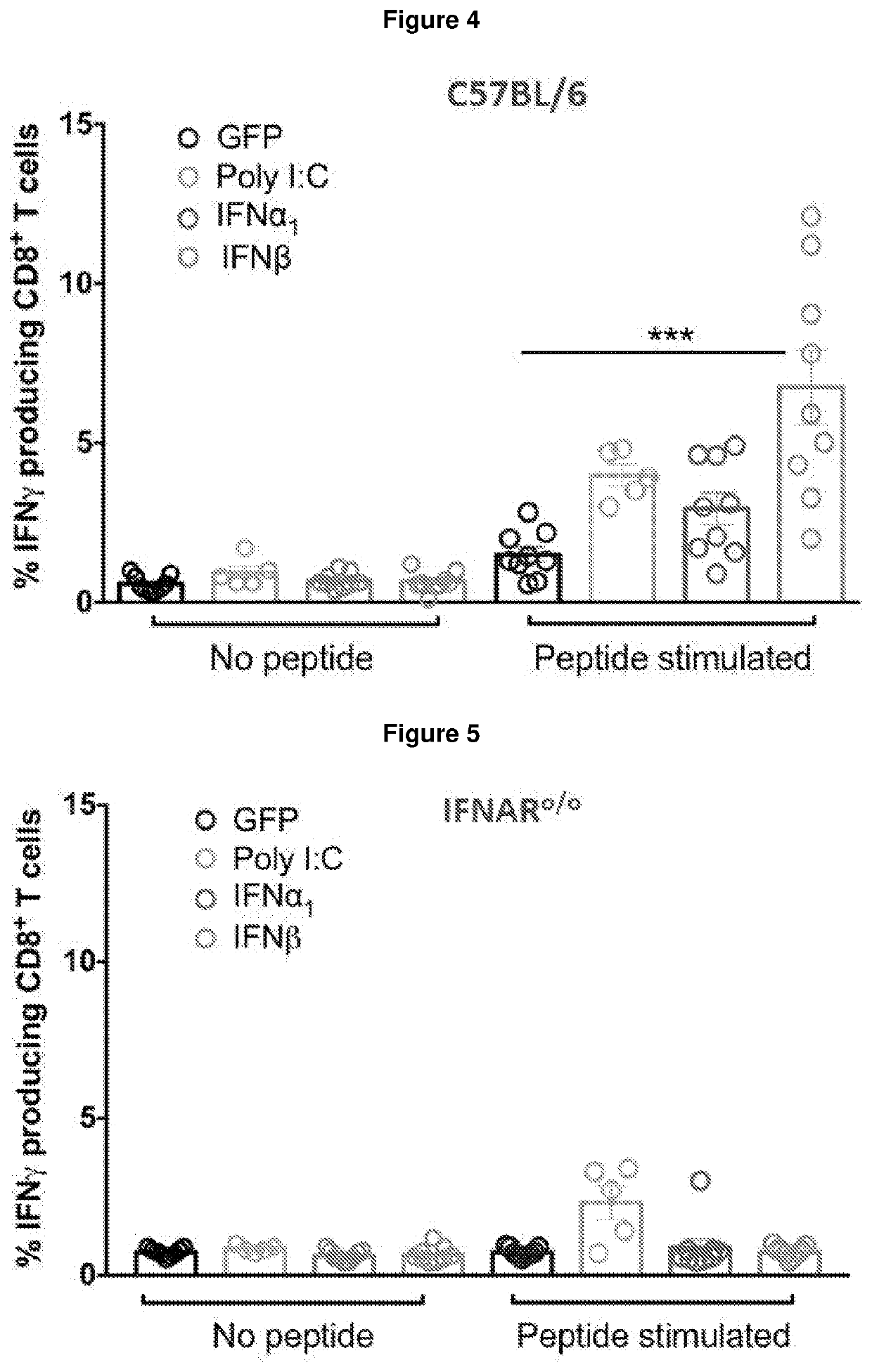
The present invention provides an immunogenic composition comprising, consisting essentially of or consisting of: a) a cell expressing an interferon-β (IFN-β) receptor agonist, and b) a tumour antigen. The invention also provides methods of treatment using said compositions.
Owner:TELETHON KIDS INST
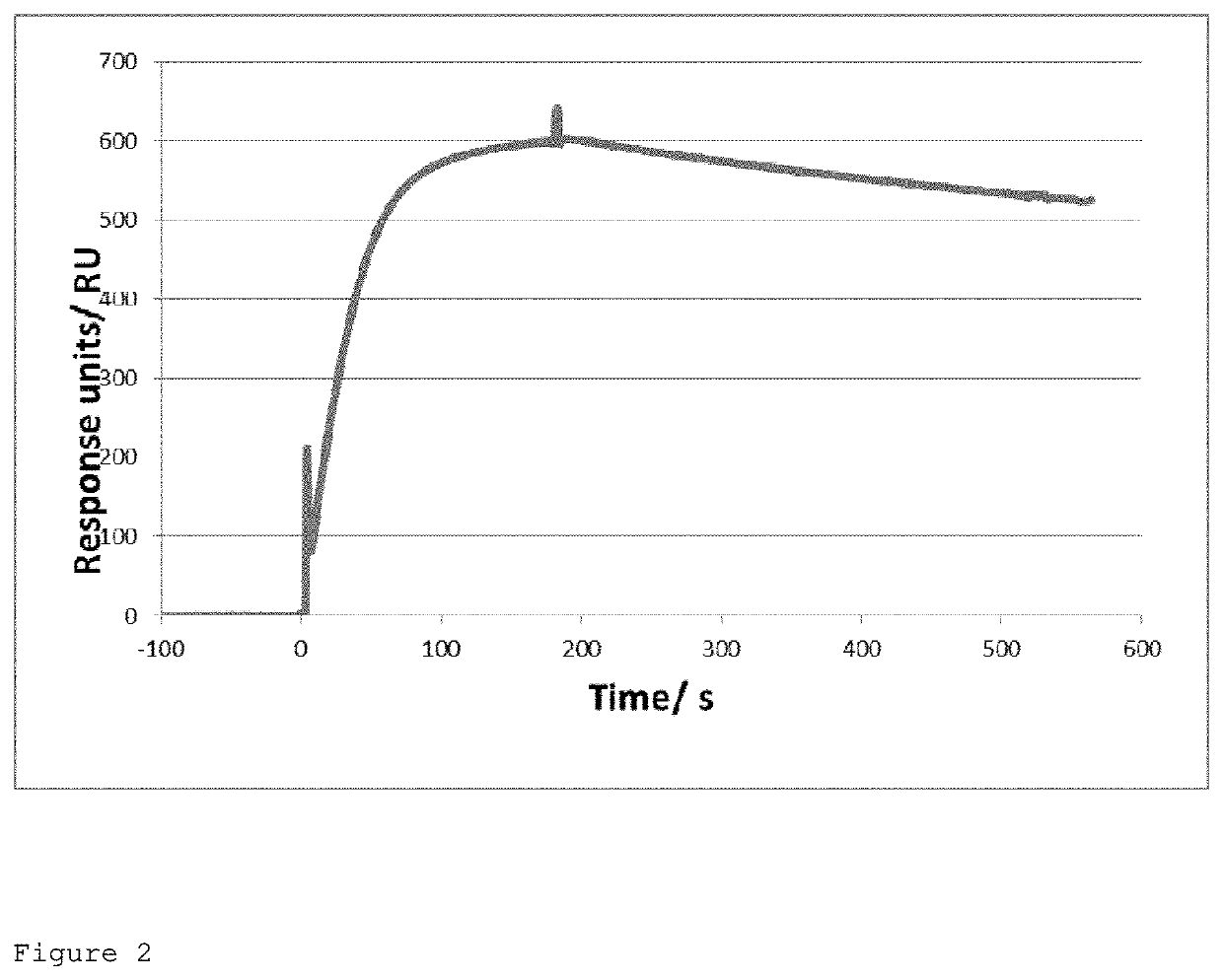
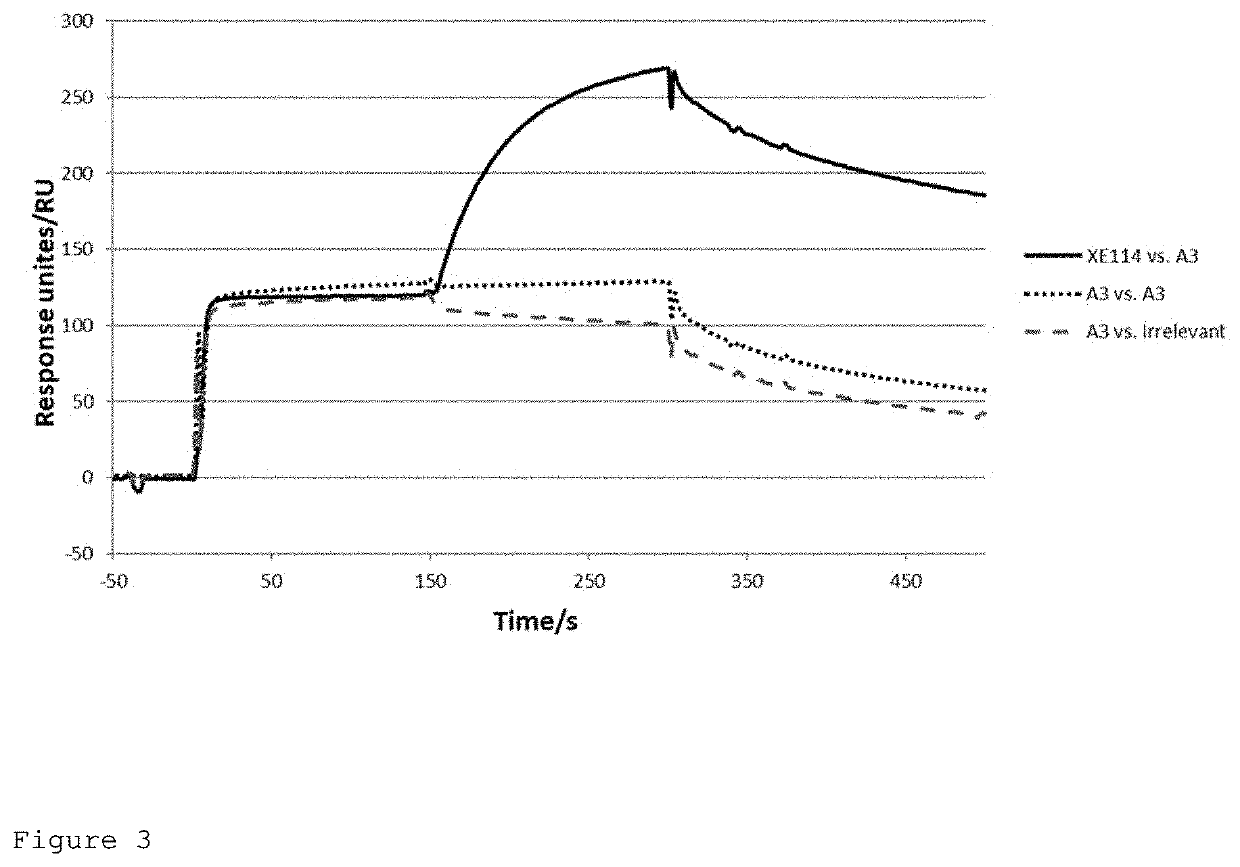
Antibodies To Tumour Antigens
ActiveUS20210054095A1Improve toleranceLow toxicityTumor necrosis factorImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesTumor solid
The present application relates to specific binding members that bind carbonic anhydrase IX (CAIX). In particular, the present application relates to the treatment, diagnosis and detection of tumours, e.g. solid tumours, using specific binding members that bind CAIX. The specific binding member may be conjugated to a biocidal or cytotoxic molecule, or to a detectable label.
Owner:PHILOGEN SRL LLC
Tumour antigen protein and tumour antigen peptide
A tumor antigen protein and its tumor antigen peptide used to prepare the medicine for treating liver cancer are disclosed. Said tumor antigen protein can generate the peptide fragment by cell endolysis. Said peptide fragment can bind with the (MHC)-I molecular compatible with main tissue and can be recognized by T cells. The DNA sequence coding said tumor antigen protein has the nucleotide sequence TSPYNM 003308 in GenBank.
Owner:PEKING UNIV
L2A5 antibody or functional fragment thereof against tumour antigens
ActiveUS11353460B2Polypeptide with localisation/targeting motifMicroorganismsAntibody fragmentsNucleotide
This invention provides an antibody or functional antibody fragments, or probe thereof directed against a unique group of antigens identified in cancer.The present invention comprises nucleotide sequences derived from L2A5 monoclonal antibody. The antibody or functional antibody fragment, or probe thereof includes a variable heavy chain domain and a variable light chain domain that has an amino acid sequence provided herein. This DNA / amino acid sequence conjugation is unique and has never been described before. The present invention further provides antibody or functional antibody fragment or a conjugate or a recombinant protein useful in the detection, treatment and prevention of human disease, including cancer.
Owner:UNIV NOVA DE LISBOA +2
Anti-cancer composition comprising proline or its derivatives and an anti-tumour antibody
The present invention relates to a pharmaceutical composition comprising of proline or proline derivatives or their salts, esters, isomers, racemates, enantiomeres or prodrugs together with an anti-cancer ligand, preferably an antibody directed to a tumour antigen. The invention is also directed to the use of proline or proline derivatives or their salts, esters, isomers or prodrugs for the manufacture of a pharmaceutical composition for treating cancer and to a method of cancer treatment by administering said composition.
Owner:SALAMA ZOSER B
Compound of prostate stem cell antigen polypeptide and nucleic acid and preparation method and application thereof
InactiveCN102294025BFree from degradationImproving immunogenicityGenetic material ingredientsPharmaceutical non-active ingredientsT lymphocytePeptide vaccine
The invention discloses a compound of PSCA (Prostate Stem Cell Antigen) polypeptide and nucleic acid, which is a granular compound obtained by the way that through positive and negative charge attraction, a PSCA gene recombination eukaryotic expression vector is packaged by fusogenic peptide formed by a positive ion peptide DNA (Deoxyribonucleic Acid) transport carrier and HLA-A2 limit CTL (Cytotoxic T Lymphocyte) epitope peptide PSCA 14-22. The preparation method comprises the steps that in buffer salt solution with pH of 7.2-7.6, the fusogenic peptide and the PSCA gene recombination eukaryotic expression vector are added according to a certain positive and negative charge ratio, are then mixed, and are left standing under room temperature for 0.5-1.5 hours to obtain the compound; the compound is not only combined with the advantages of DNA vaccine and epitope peptide vaccine, but also can imitate natural virus to directly 'infect' APC (Antigen Presenting Cell) and other immune cells, so as to stimulate strong CTL response in vivo. The compound is expected to break down selftolerance of tumour antigen, can be used to prepare prostate cancer therapeutic vaccine of more than 50 % of population in China, and has potential and good development and application prospect in the prostate cancer immunization therapy field.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com