Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
58 results about "HY Antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
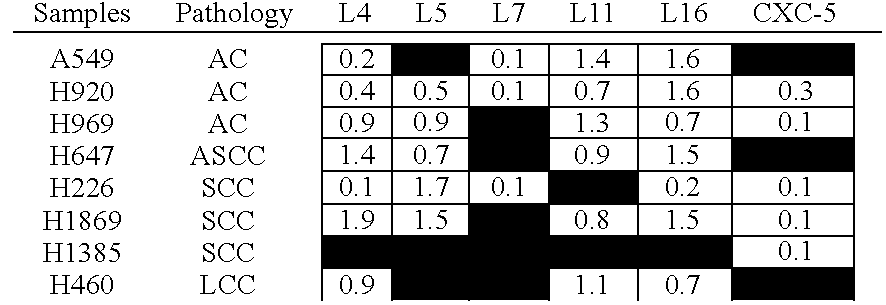
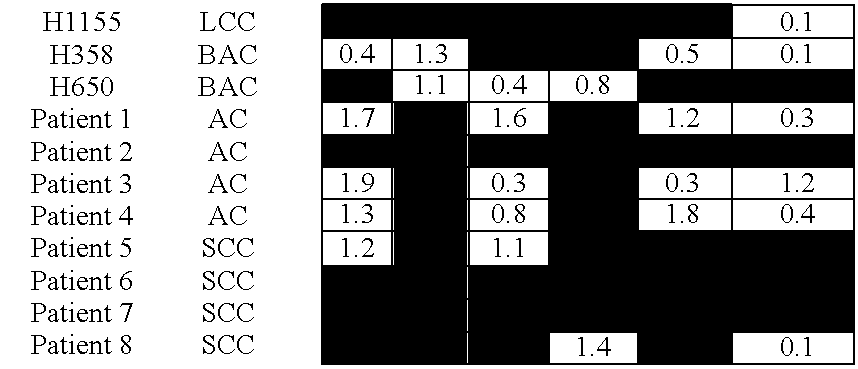
Expression profiling in non-small cell lung cancer
InactiveUS20050272061A1Modulate expressionPreventing and delaying onsetMicrobiological testing/measurementMaterial analysisAntigenFhit gene
The present invention relates to L genes and gene products that are differentially expressed in cancer tissues and cell lines. In a particular aspect of the invention, L genes and gene products are differentially expressed in lung cancer tissues and cell lines. In accordance with the present invention, L nucleic acid sequences, amino acid sequences and antibodies thereto, and methods of use thereof are presented. The L molecules and methods of the invention may be used to monitor expression levels of L genes, wherein the detection of aberrant levels of L molecules provides a positive diagnostic indicator of lung cancer and / or other L gene associated cancers and a useful prognostic indice of the state of such diseases. Also provided are compounds capable of modulating an L molecule mediated activity, which are identified using the L molecules and methods of the invention. Such L molecule modulating compounds may be used efficaciously to treat patients with lung cancer, or other L antigen positive cancers.
Owner:SEATTLE GENETICS INC
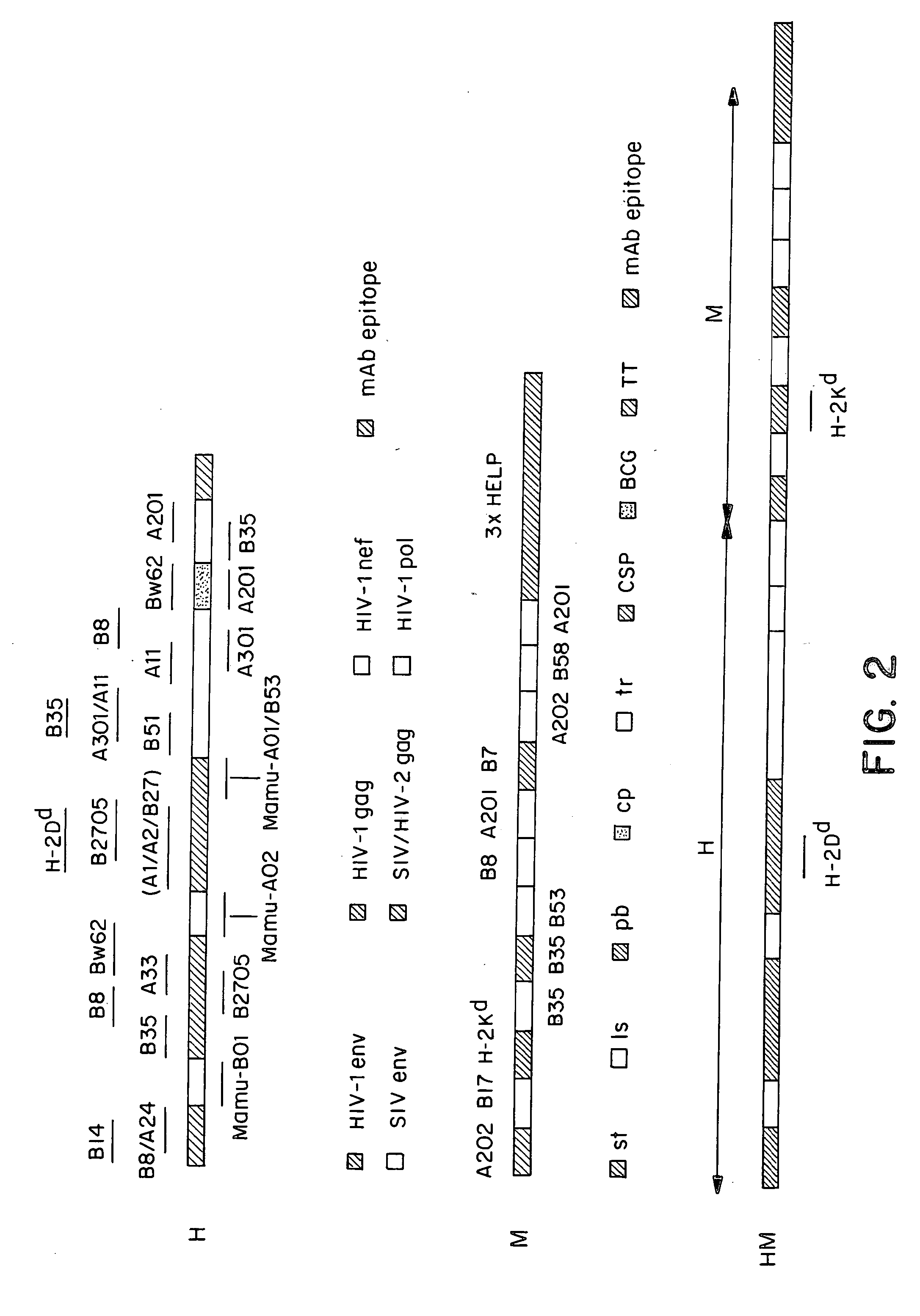
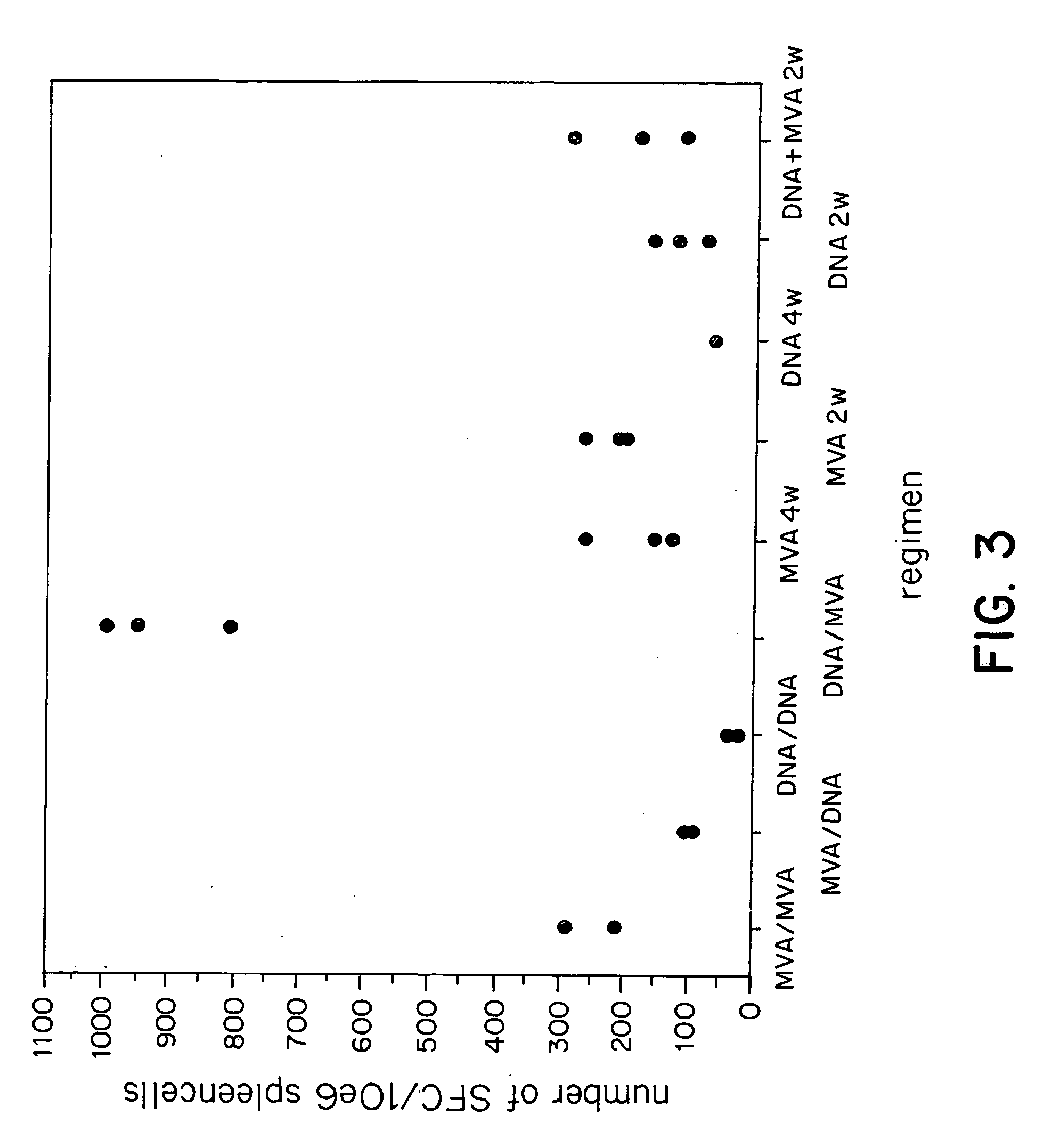
Methods and reagents for vaccination which generate a CD8 T cell immune response
New methods and reagents for vaccination are described which generate a CD8 T cell immune response against malarial and other antigens such as viral and tumour antigens. Novel vaccination regimes are described which employ a priming composition and a boosting composition, the boosting composition comprising a non-replicating or replication-impaired pox virus vector carrying at least one CD8 T cell epitope which is also present in the priming composition.
Owner:OXXON THERAPEUTICS LTD
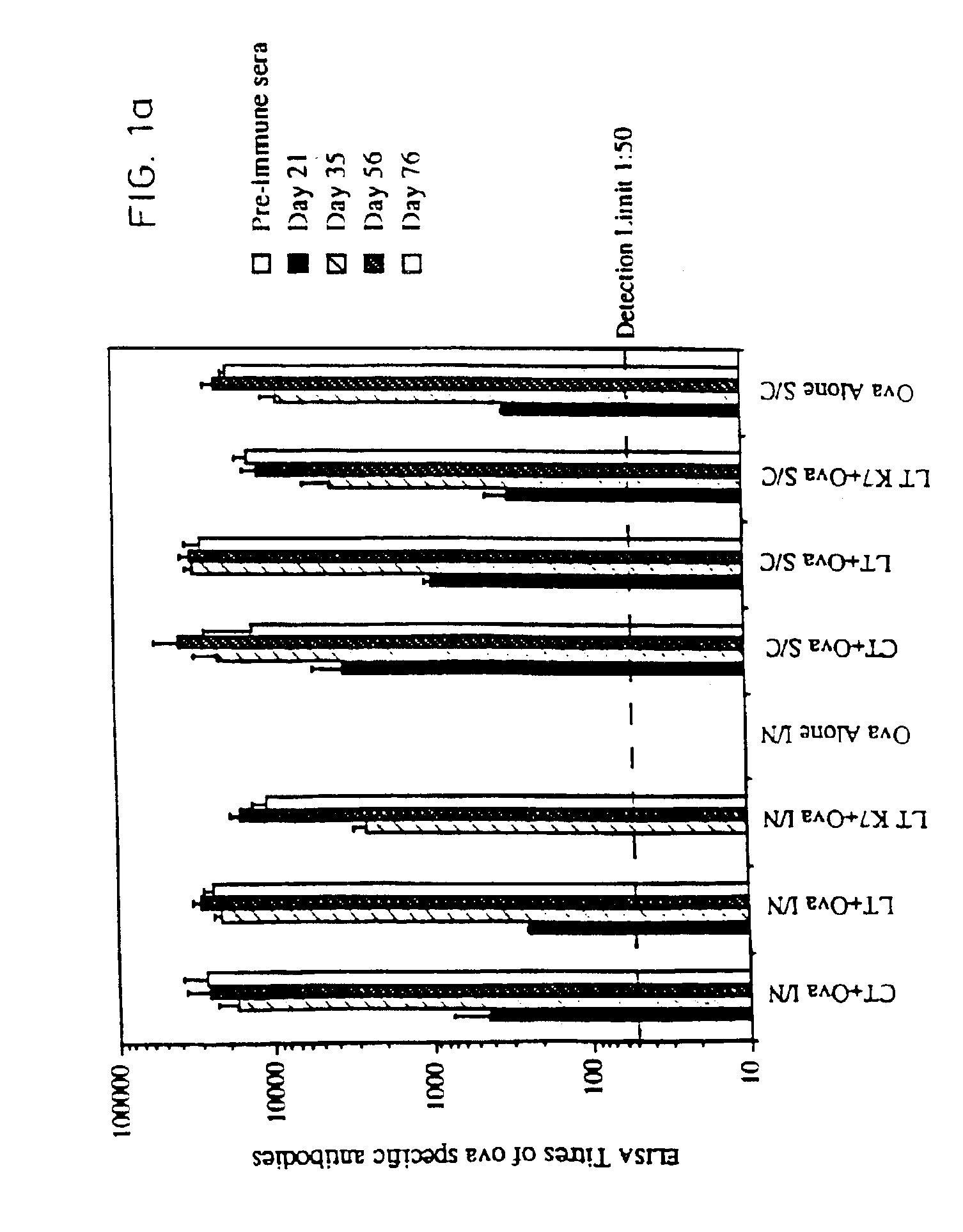
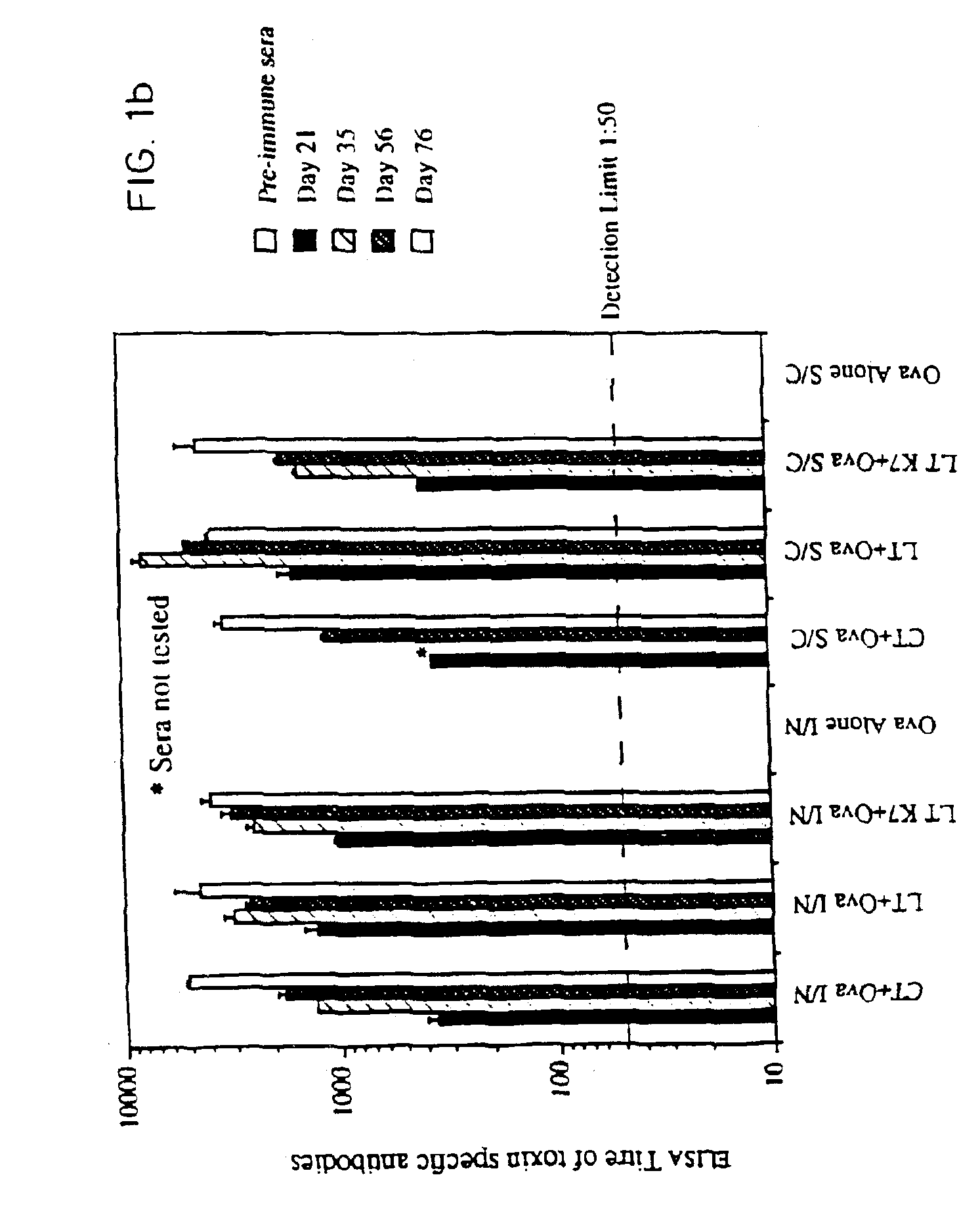
Nontoxic mucosal adjuvant
InactiveUS7070781B2Improving immunogenicityImprove responseAntibacterial agentsBiocideBiological bodyGynecology
A non-toxic mucosal adjuvant is provided which may be admixed with further antigens to provide a vaccine administrable to mucosal surfaces in organisms including man. Preferably, the non-toxic mucosal adjuvant is a detoxified mutant of a bacterial ADP-ribosylating toxin, optionally comprising one or more amino acid additions, deletions or substitutions.
Owner:CHIRON CORP
Preparation method and device of duramater/spinal dural transplanting substitute
ActiveCN102727935ASimple Surface Functional StructureWidely sourced and cheapProsthesisAntigenDefect repair
The invention provides a preparation method of a duramater / spinal dural transplanting substitute which is obtained by repeated freezing and thawing of dural tissue, rolling and cracking of cells, crosslinking fixed protection, accellular antigen extraction, dense surface fibrosis modification, packaging and sterilization, and has the advantages of simple method, wide raw material sources, cheap raw materials, and low cost. The prepared dural substitute completely removes components of cells and other antigen components simultaneously when protecting dural tissue natural structure and properties, is good in biocompatibility, free of immune rejection, safe and reliable, good in mechanical performance, and easy in clinical operation, can meet the needs of defect repair, has the function of promoting tissue regeneration as a loose surface is beneficial to the tissue fluid adsorption, active factor enrichment, and growth of blood vessels and cells, and has the advantages of rapidness in fusion with a host, biodegradable absorption, and good repair effect. The animal test shows that the defect can be completely repaired without brain or spinal fluid leakage, or adhesion with brain tissue, and significant rejection is not found.
Owner:SHAANXI BIO REGENERATIVE MEDICINE CO LTD
Mucosal vaccines with chitosan adjuvant and meningococcal antigens
InactiveUS20060051378A1Improve securityReduced dosAntibacterial agentsOrganic active ingredientsSalmonella serotype typhiCarrier protein
The invention provides immunogenic compositions comprising (a) a capsular saccharide antigen from serogroup C of N. meningitidis, and (b) a chitosan adjuvant. The composition preferably comprises (c) one or more further antigens and / or (d) one or more further adjuvants. The compositions are particularly suitable for mucosal delivery, including intranasal delivery. The invention also provides immunogenic compositions for mucosal delivery comprising capsular saccharides from at least two of serogroups A, C, W135 and Y of N. meningitidis. It is preferred that the capsular saccharides in the compositions of the invention are conjugated to carrier protein(s) and / or are oligosaccharides. Conjugated oligosaccharide antigens are particularly preferred.
Owner:NOVARTIS AG +1
Immunogenic compositions for streptococcus pyogenes
ActiveUS7709009B2Antibacterial agentsAntibody mimetics/scaffoldsStreptococcus halichoeriBiochemistry
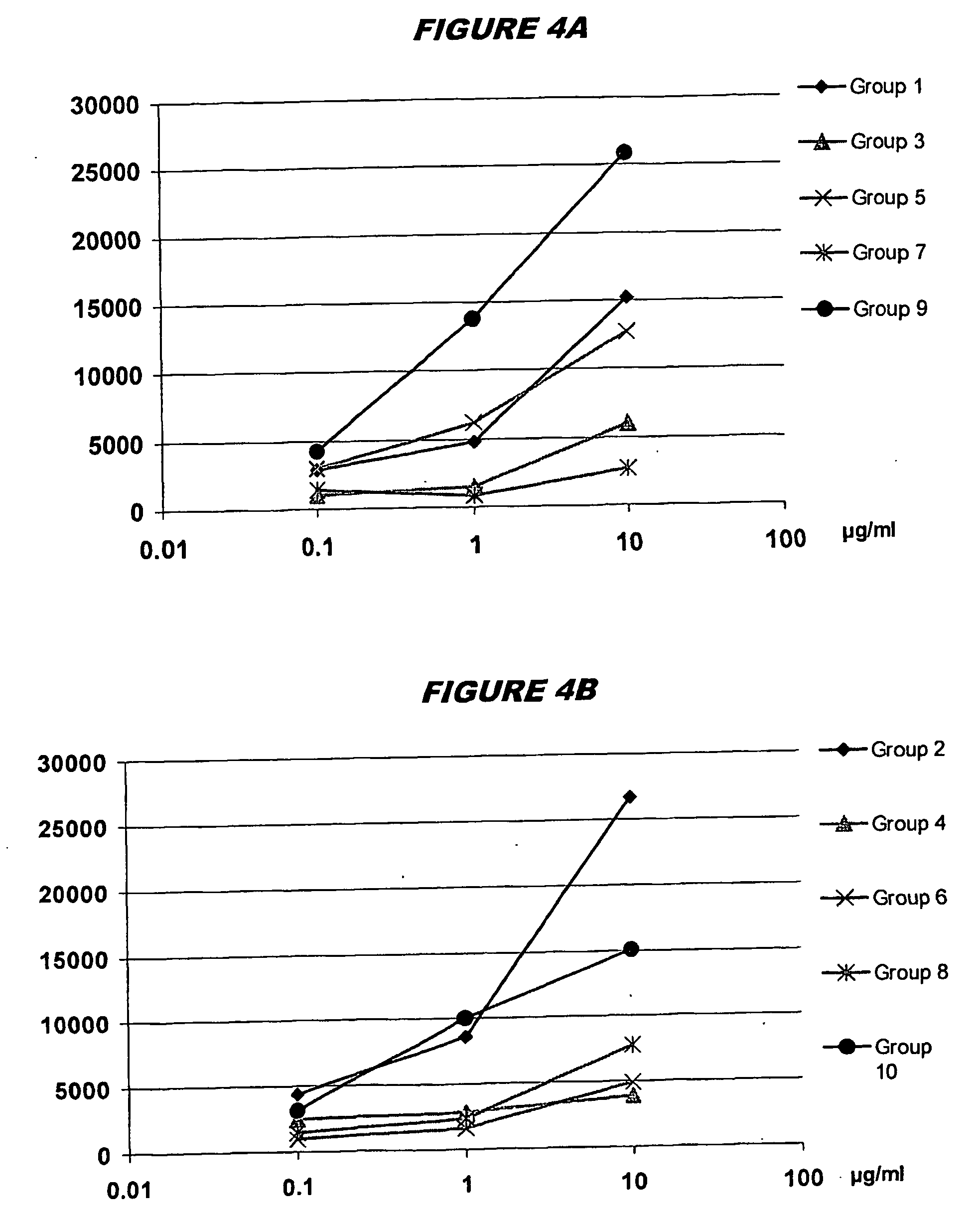
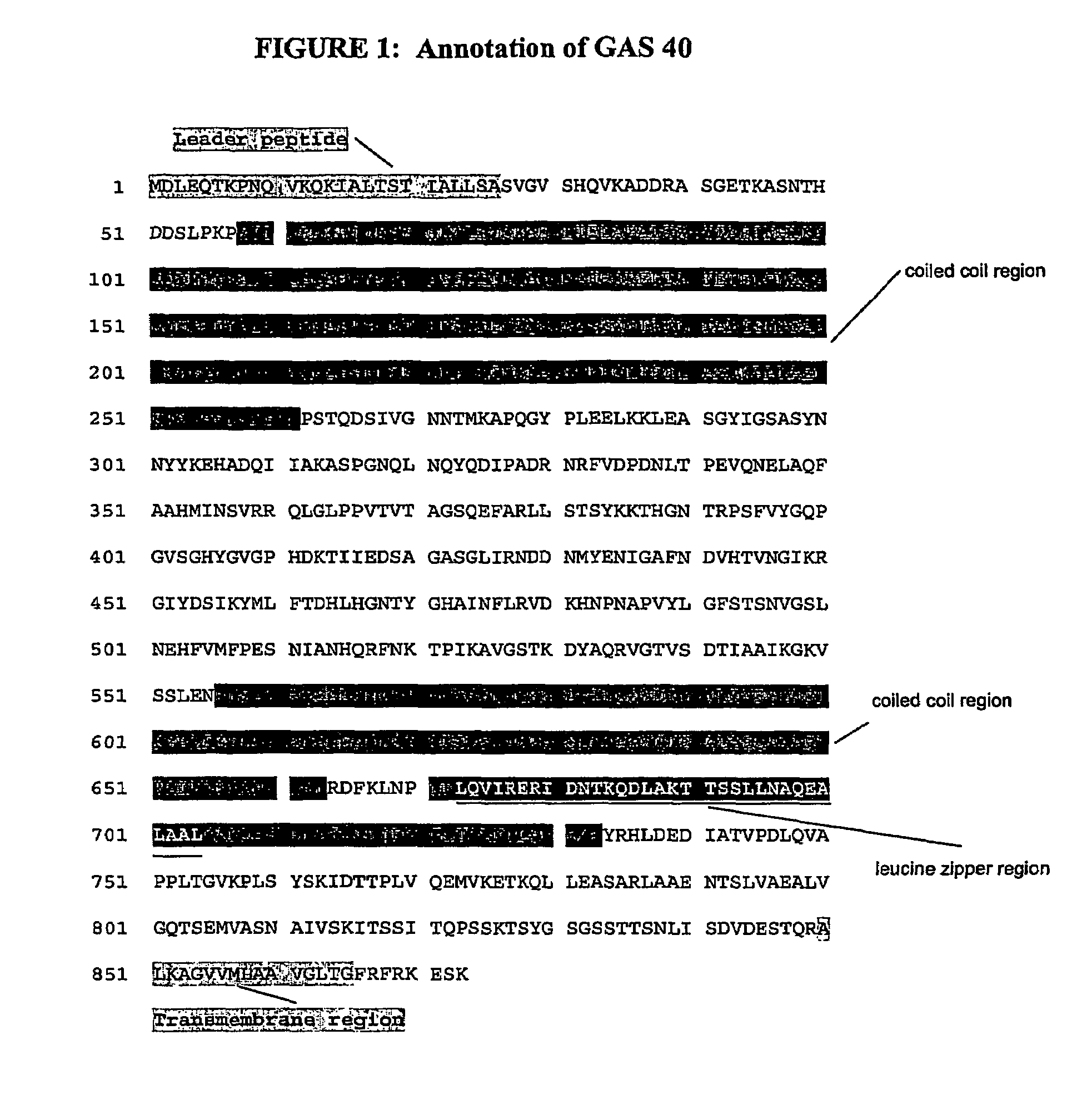
The invention includes a GAS antigen, GAS 40, which is particularly suitable for use either alone or in combinations with additional GAS antigens, such as GAS 117, GAS 130, GAS 277, GAS 236, GAS 40, GAS 389, GAS 504, GAS 509, GAS 366, GAS 159, GAS 217, GAS 309, GAS 372, GAS 039, GAS 042, GAS 058, GAS 290, GAS 511, GAS 533, GAS 527, GAS 294, GAS 253, GAS 529, GAS 045, GAS 095, GAS 193, GAS 137, GAS 084, GAS 384, GAS 202, and GAS 057.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
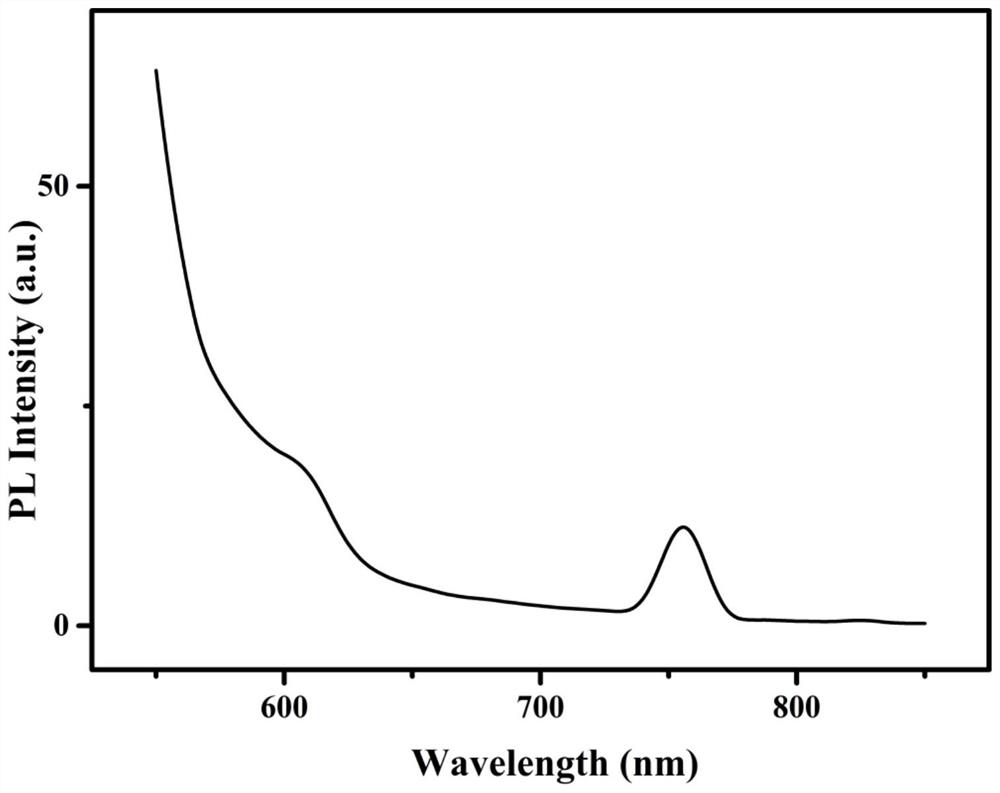
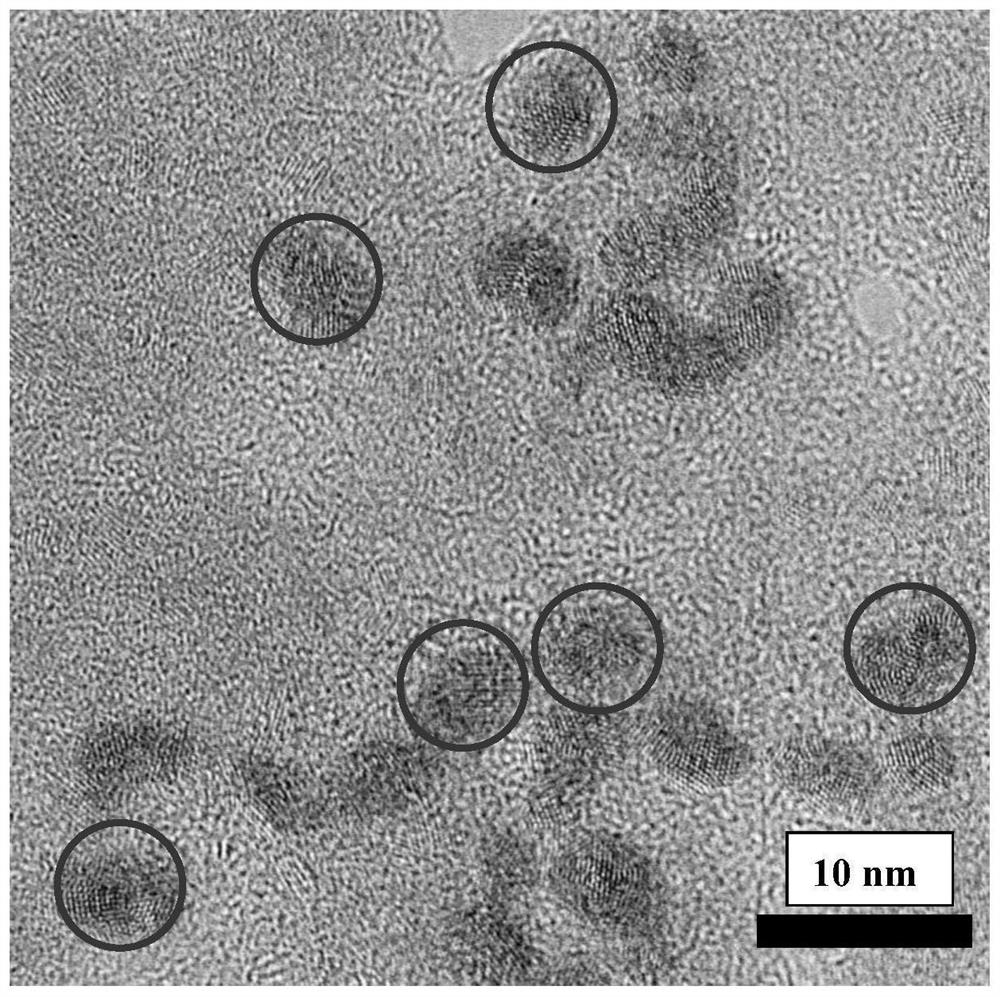
Preparation method of spectral near-infrared electrochemical luminescence immunosensor
ActiveCN112147132ANo distractionHigh selectivityChemiluminescene/bioluminescenceMaterial electrochemical variablesImmunityElectrochemiluminescence
The invention belongs to the field of analysis technical methods, and relates to preparation of a spectral near-infrared electrochemical luminescence (915nm) immunosensor. The preparation method mainly comprises the following steps that (1), methionine is used as a stabilizer to prepare a water-soluble Au-Ag bimetallic nanocluster capable of generating near-infrared electrochemical luminescence; (2), a gold-silver bimetallic nanocluster-marked secondary antibody (Au-AgAb2) is prepared; (3), a spectral near-infrared electrochemical luminescence sensor is prepared by taking the gold-silver bimetallic nano gold-silver cluster as a marker on the basis of a sandwich immunity principle; and (4), a working curve is drawn. The spectral electrochemical luminescence immunosensor is high in detectionsensitivity, and other antigen proteins do not interfere with the sensing detection of the target antigen.
Owner:SHANDONG UNIV
Multi-component vaccine comprising at least three antigens to protect against disease cased by Haemophilus influenzae
InactiveUS6342232B1No suppression of anti-rHMW responseStimulate immune responseAntibacterial agentsSenses disorderProteolysisDiphtheria vaccination
A multi-component immunogenic composition confers protection on an immunized host against infection caused by Haemophilus influenzae. Such composition comprises at least three different antigens of Haemophilus influenzae, two of which are adhesins. High molecular weight (HMW) proteins and Haemophilus influenzae adhesin (Hia) proteins of non-typeable Haemophilus influenzae comprise the adhesin components while the other antigen is a non-proteolytic analog of Hin47 protein. Each component does not impair the immunogenicity of the others. The Haemophilus vaccine may be combined with DTP component vaccines, which may contain inactivated poliovirus, including type 1, type 2 and / or type 3, and / or a conjugate of a capsular polysaccharide of Haemophilus influenzae and tetanus or diphtheria toxoid, including PRP-T, to provide a multi-valent component vaccine without impairment of the immunogenic properties of the other antigens.
Owner:AVENTIS PASTUER LTD
Cattle and sheep brucellosis indirect enzyme-linked immunosorbent assay antibody detection kit and preparation method thereof
InactiveCN103543261AIncreased sensitivityStrong specificityBiological material analysisEscherichia coliBiology
The invention relates to a cattle and sheep brucellosis indirect enzyme-linked immunosorbent assay antibody detection kit and a preparation method thereof, belongs to the field of detection of pathogens of zoonotic infections and aims at solving the problems of low sensitivity, poor specificity and the like in detection of Brucella by adopting other antigens. The kit is assembled by taking a recombinant Brucella BCSP31 protein with expression induced by SUMO as an expression vector in Escherichia coli to serve as an antigen-coated ELISA (enzyme-linked immunosorbent assay) plate, adding serum to be detected and rabbit anti-bovine HRP-IgG or rabbit anti-goat HRP-IgG and adding a washing buffer solution, a blocking solution, a TMB (3,3',5,5'-Tetramethylbenzidine) primer solution and a stop solution for matching. The prokaryotic expression vector, namely the SUMO, is utilized to express the Brucella BCSP31 protein in an efficient and soluble manner, the kit is used for establishing a Brucella ELISA detection method, the detection has better repeatability and specificity, and cattle and sheep Brucella can be fast and efficiently detected by utilizing the method.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS +1
SPA-antibody tripolymer, cell treating kit containing tripolymer, preparation method and application thereof
InactiveCN101799473ASimple methodLow costBlood/immune system cellsBiological testingProgenitorRed blood cell
The invention relates to an SPA-antibody tripolymer, a cell treating kit containing the tripolymer, a preparation method and application thereof. The tripolymer comprises an anti erythrocyte antibody, an SPA and an antibody of a certain anti leukocyte antigen or other antigens. The tripolymer is combined with an erythrocyte through the anti erythrocyte antibody per se, the other antibody is combined with a corresponding leukocyte antigen (or other antigens), and then leukocyte (other cells, factors) and the erythrocyte are deposited through an erythrocyte sedimentation process or other methods of density gradient centrifugation and the like, thereby achieving the purpose of eliminating ingredients of corresponding cells and the like, connecting the erythrocyte with a corresponding antigen by the SPA, and being used as a mean for detecting a certain antigen. The invention has convenience and simpleness, and can be directly used for clinically separating and extracting stem cells / progenitor cells; and the collected and extracted stem cells / progenitor cells have no external markers. Meanwhile, the kit based on the tripolymer can be industrially produced so as to realize the popularization of the separation and purification technology of hematopoietic stem cells.
Owner:王信
Anti-PD-L1/anti-LAG3 bispecific antibody and uses thereof
ActiveCN110678484AHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsHumaninAntiendomysial antibodies
The invention provides an anti-PD-L1 / anti-LAG3 bispecific antibody, which can effectively block the interaction between PD-L1 and PD-1 and the interaction between LAG3 and a ligand (MHC II molecules,FGL1, etc.). The bispecific antibody is highly affinity to PD-L1 proteins (human PD-L1 protein, for example) and LAG3 proteins (human LAG3 protein, for example). The invention also provides an antibody and segment that has specificity on PD-L1 or LAG3 proteins; or an antibody and segment that has specificity on one or more other antigens.
Owner:I MAB BIOPHARMA HANGZHOU CO LTD
Non-toxic mucosal adjuvant
InactiveUS20060177469A1Improving immunogenicityImprove responseAntibacterial agentsBacterial antigen ingredientsMucosal adjuvantMutant
A non-toxic mucosal adjuvant is provided which may be admixed with further antigens to provide a vaccine administrable to mucosal surfaces in organisms including man. Preferably, the non-toxic mucosal adjuvant is a detoxified mutant of a bacterial ADP-ribosylating toxin, optionally comprising one or more amino acid additions, deletions or substitutions.
Owner:CHIRON CORP
H7N9 subtype avian influenza genetic engineering vaccine taking baculovirus as carrier as well as preparation method and application of vaccine
InactiveCN106421771AEasy to makeEase of mass productionSsRNA viruses negative-senseViral antigen ingredientsAntigenProtective antigen
The invention discloses an H7N9 subtype avian influenza genetic engineering vaccine taking baculovirus as a carrier as well as a preparation method and an application of the vaccine and belongs to the technical field of genetic engineering. The H7N9 subtype avian influenza genetic engineering vaccine takes the baculovirus as the carrier and is a recombinant baculovirus capable of expressing main protective antigens of the avian influenza virus in vertebrate. The avian influenza genetic engineering vaccine realizing immunity through intramuscular injection is prepared. The vaccine overcomes defects that inactivated vaccines depend on chicken embryo production, the production period is long, other antigen mixed infection exists and the like, and technical reserve is provided for screening the H7N9 subtype avian influenza genetic engineering vaccine.
Owner:SOUTH CHINA AGRI UNIV
Method of stimulating an immune response by administration of host organisms that express intimin alone or as a fusion protein with one or more other antigens
InactiveUS6881411B2Improve bindingImprove complianceAntibacterial agentsOrganic active ingredientsBacteroidesEscherichia coli
This invention satisfies needs in the art by providing intimin, the Enterohemorrhagic Escherichia coli (EHEC) adherence protein, alone or as a fusion protein with one or more other antigens, expressed by transgenic plants and the use of those plants as vehicles for stimulating a protective immune response against EHEC and the one or more other antigens. Various plant species are transformed to protect various animal species and also humans against EHEC, against pathogens expressing intimin-like proteins, and against pathogens expressing any of the one or more other antigens to which intimin may be fused.The eae gene encoding intimin, a functional portion thereof, or a recombination that encodes a fusion protein is put under the control of a constitutive plant promoter in a plasmid and the plasmid is introduced into plants by the type of transformation appropriate for the particular plant species. The engineered plants expressing intimin or the intimin fusion protein are then fed to animals and / or humans to elicit the production of antibodies, which protect the animals / humans against EHEC colonization and infection, and against pathogens expressing the one or more other antigens and any cross-reactive antigens. The invention may also be practiced by expressing the intimin or intimin fusion protein in other host organisms such as bacteria, yeast, and fungi.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
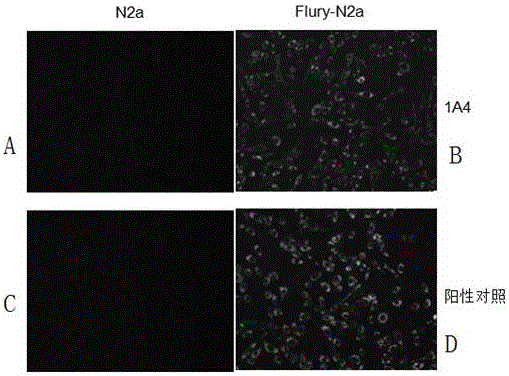
Hybridoma cell strain and rabies virus phosphoprotein monoclonal antibody generated by same
ActiveCN106701687AStrong specificityHigh potencyMicroorganism based processesImmunoglobulins against virusesWestern blotAscites
The invention provides a hybridoma cell strain 1A4. The hybridoma cell strain provided by the invention can be used for secreting and preparing a monoclonal antibody capable of effectively identifying rabies virus phosphoprotein; the titer of a mouse ascites antibody is 2*10<5> measured by indirect enzyme-linked immunosorbent assay; the monoclonal antibody does not conduct cross reaction with other proteins of rabies virus, other antigens and pathogens, and has the advantages of high specificity and high sensitivity and can be applied to the biological diagnosis of rabies virus by ELISA, Western-blot and immunofluorescence and has good application prospect.
Owner:KUNMING UNIV OF SCI & TECH
Rabies virus nucleoprotein monoclonal antibody and application thereof
ActiveCN105504051AHigh specificityHigh sensitivityImmunoglobulins against virusesMicroorganism based processesCombined VaccinesHeavy chain
The invention provides a rabies virus nucleoprotein monoclonal antibody and application thereof. The amino acid sequence of a light chain variable region of the monoclonal antibody is shown as SEQ ID NO.1, and the amino acid sequence of a heavy chain variable region of the monoclonal antibody is shown as SEQ ID NO.2; or the amino acid sequence of the light chain variable region of the monoclonal antibody is shown as SEQ ID NO.3, and the amino acid sequence of the heavy chain variable region of the monoclonal antibody is shown as SEQ ID NO.4. The monoclonal antibody has no cross reaction with other kinds of protein of rabies viruses, other antigens and pathogens, has the advantages of high specificity and high sensitivity, can accurately detect the content of each effective component in a rabies combined vaccine in a sample and has good application prospects.
Owner:ABMAX BIOPHARMACEUTICALS
Vaccine composition comprising Flt3-ligand
InactiveUS20060292166A1Stimulate immune responseEnhance immune responseBacterial antigen ingredientsPeptide/protein ingredientsCD30 LigandAbnormal tissue growth
Flt3-ligand can be used to generate large numbers of dendritic cells from hematopoietic progenitor and stem cells. Flt3-ligand can be used to augment immune responses in vivo, and expand dendritic cells ex vivo. Such dendritic cells can then be used to present tumor, viral or other antigens to naive T cells, can be useful as vaccine adjuvants. When flt3-L is used and / or administered in combination with other reactive agents, e.g. CD40 binding proteins, 4-1BBL or antibodies reactive with 4-1BB, CD30 ligand antagonists, RANKL, and / or interferon alpha the combination further enhances immune responses and the effectiveness of vaccine adjuvants.
Owner:IMMUNEX CORP
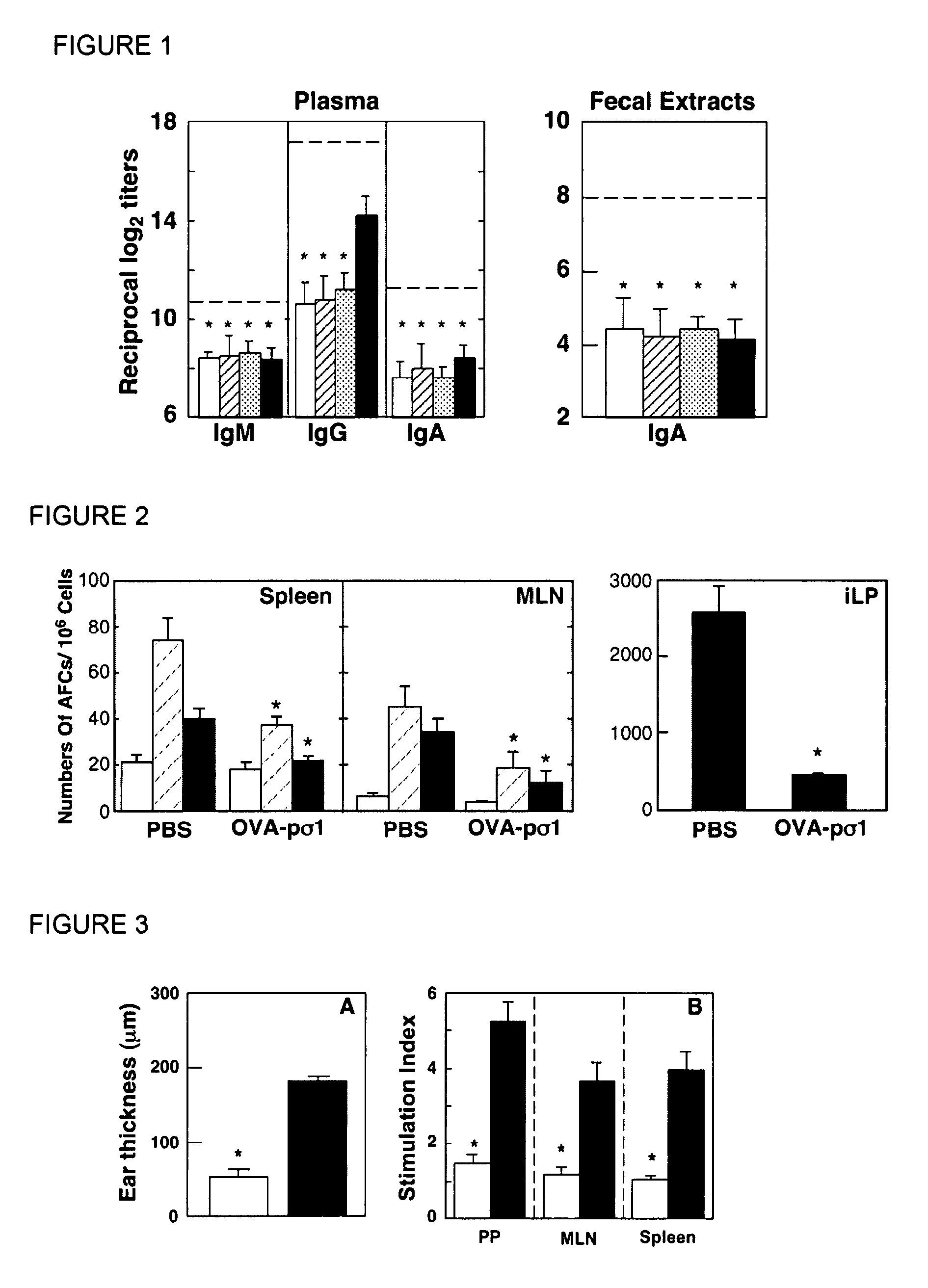
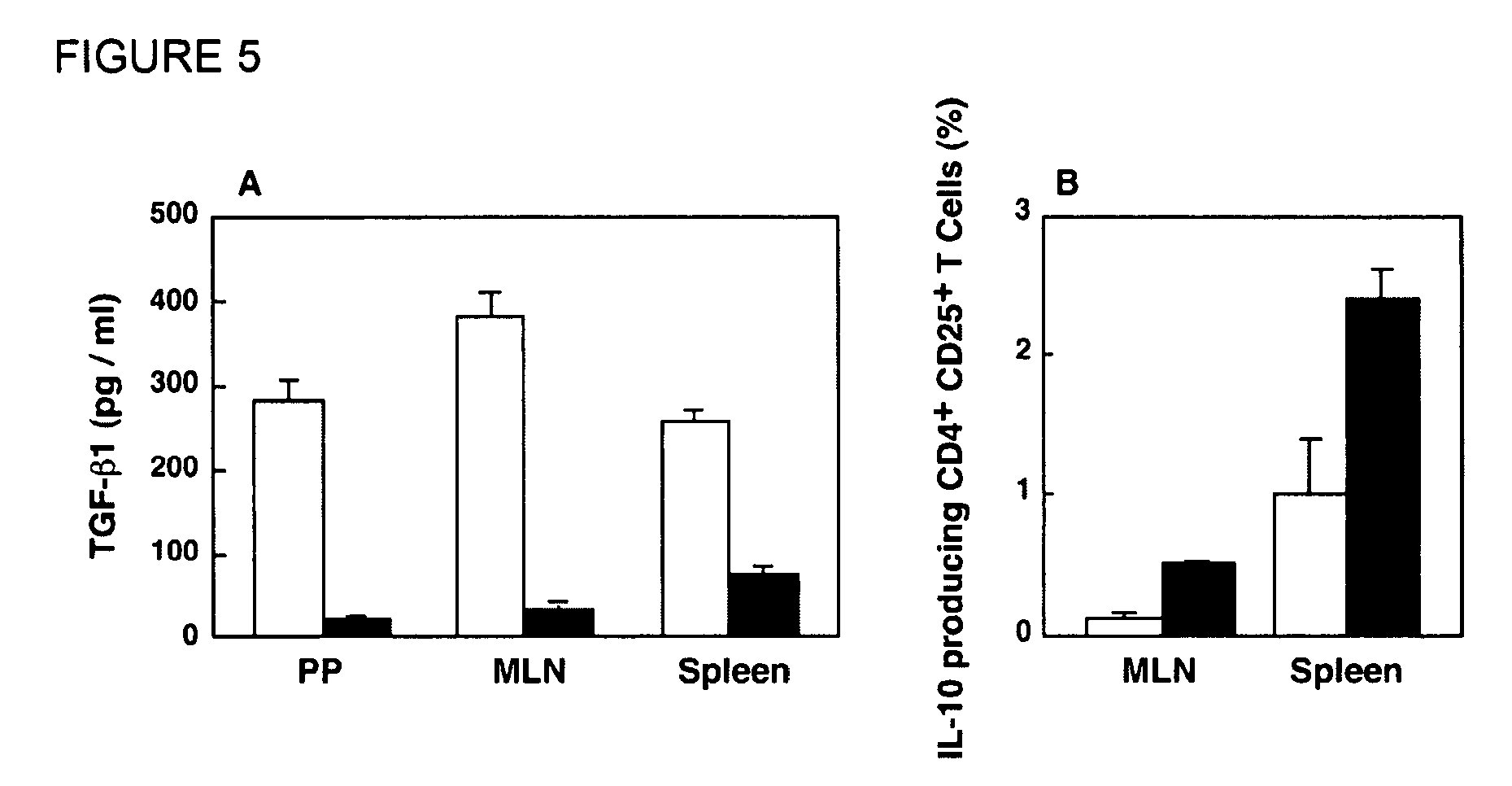
Tolerizing agents
InactiveUS20090169578A1Facilitates systemic and mucosal tolerance inductionRestoring immunogenicityNervous disorderAntipyreticNasal cavityTolerability
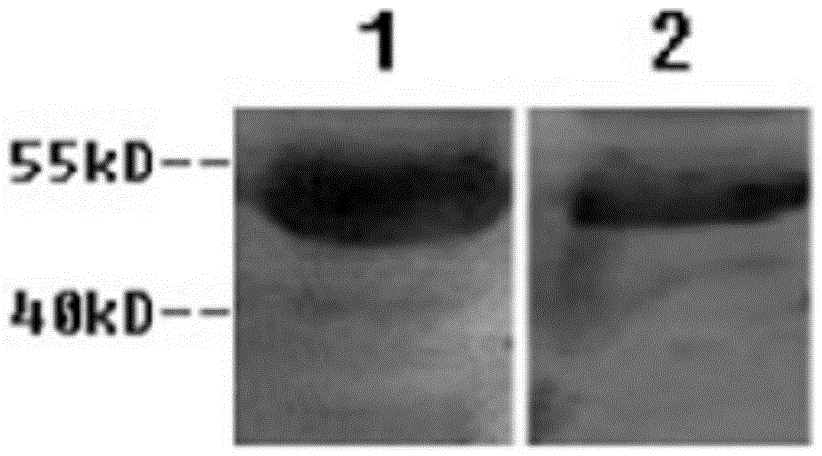
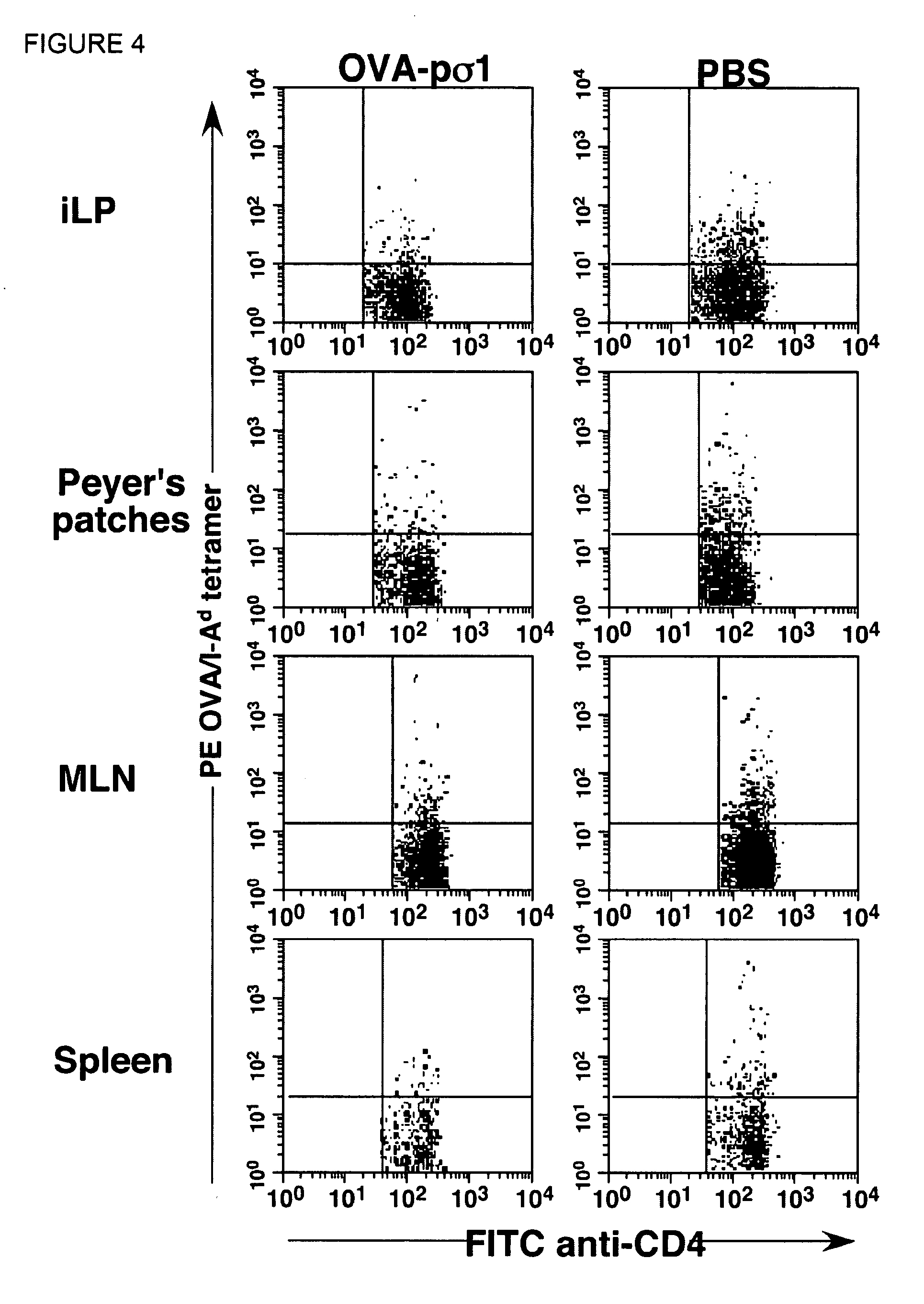
Described herein is the development of fusion proteins useful for inducing tolerance in a subject. In particular embodiments, the tolerizing agents are useful for influence autoimmune, inflammatory, and / or allergic reactions. Example tolerizing fusion proteins contain a targeting portion (which delivers the fusion protein) and a toleragen or allergen or other antigen to which tolerance is desired in a subject. In particular examples, it is demonstrated that a pσ1 fusion protein, when administered orally, facilitates systemic and mucosal tolerance. Also described is the nasal delivery of fusion proteins, for instance for restoring immunogenicity.
Owner:UAB RES FOUND +1
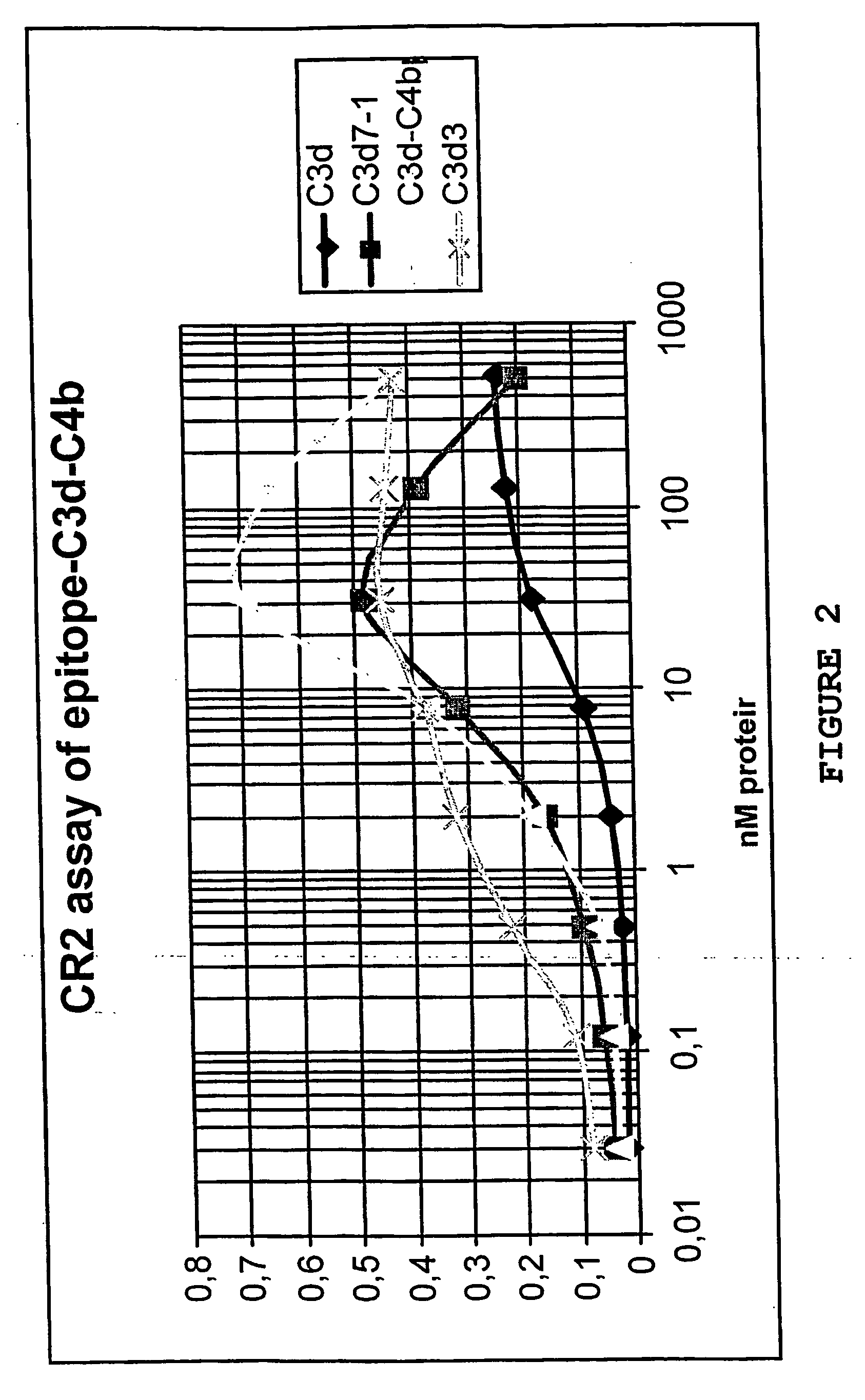
Multimeric complexes of antigens and adjuvants
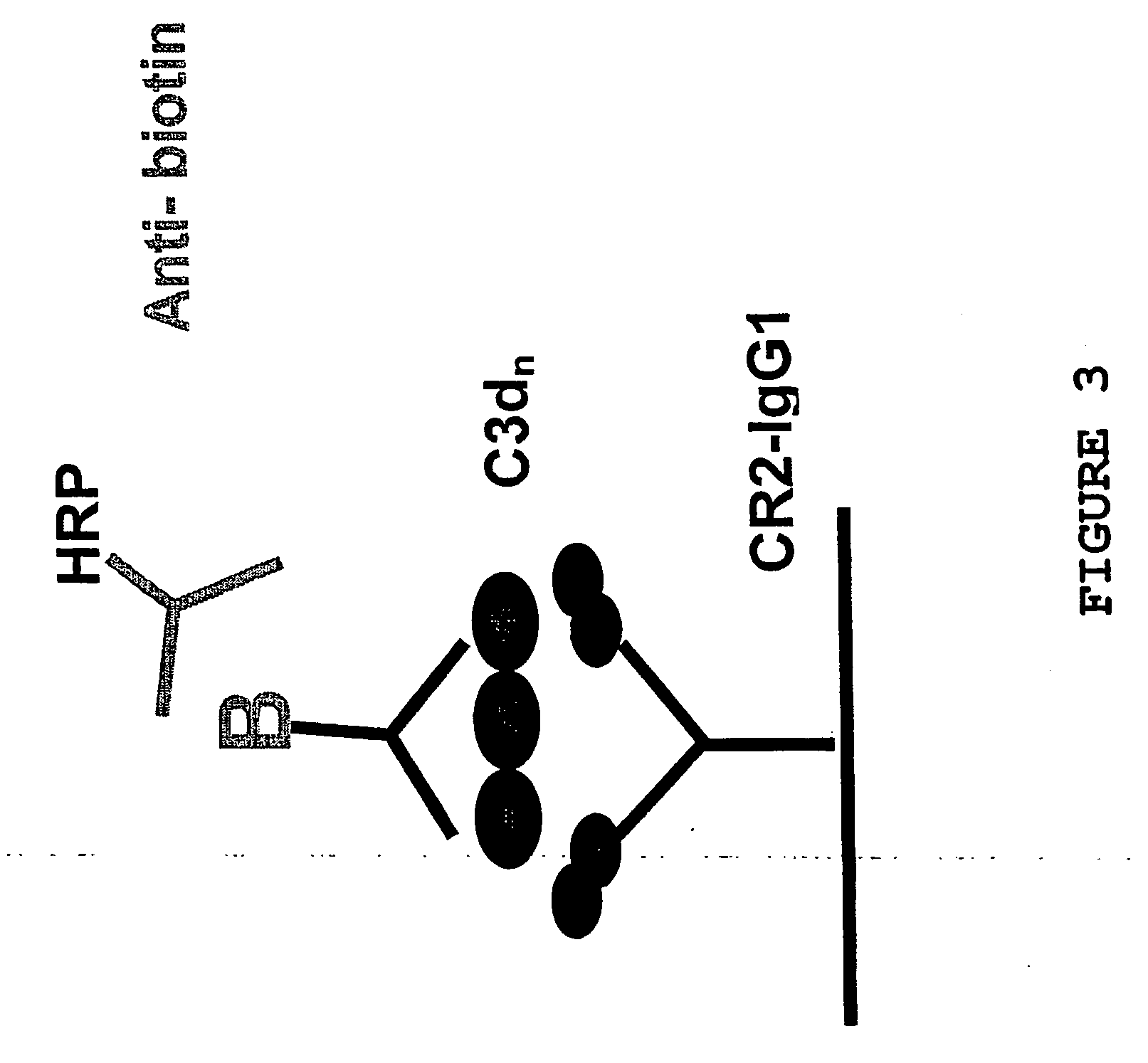
The present invention provides a product comprising: a first component which is a scaffold; a second component which is an adjuvant, preferably a polypeptide which is a ligand for CD21 or a cell surface molecule on B cells or T cells or follicular dendritic or other antigen presenting cells; and a third component which is an antigen.
Owner:AVIDIS SA
Binding agents and their use in targeting tumor cells
InactiveUS20090291075A1Powder deliveryImmunoglobulins against cell receptors/antigens/surface-determinantsDendritic cellTumor antigen
The present invention concerns methods and compositions for administering a binding agent to a patient wherein the patient generates a response to autologous tumor. The binding agents target apoptotic tumor cells and facilitates the uptake of these apoptotic tumor cell are taken up by dendritic cells or other antigen presenting cells for processing and presentation to the immune system without the expression of circulating tumor-associated antigen (or without the need of circulating tumor antigen).
Owner:ALTAREX MEDICAL
Immunogens, compositons and uses thereof, method for preparing same
InactiveUS20120328621A1Reduce intensityImproving immunogenicityProtozoa antigen ingredientsVirus peptidesAdjuvantAdult worm
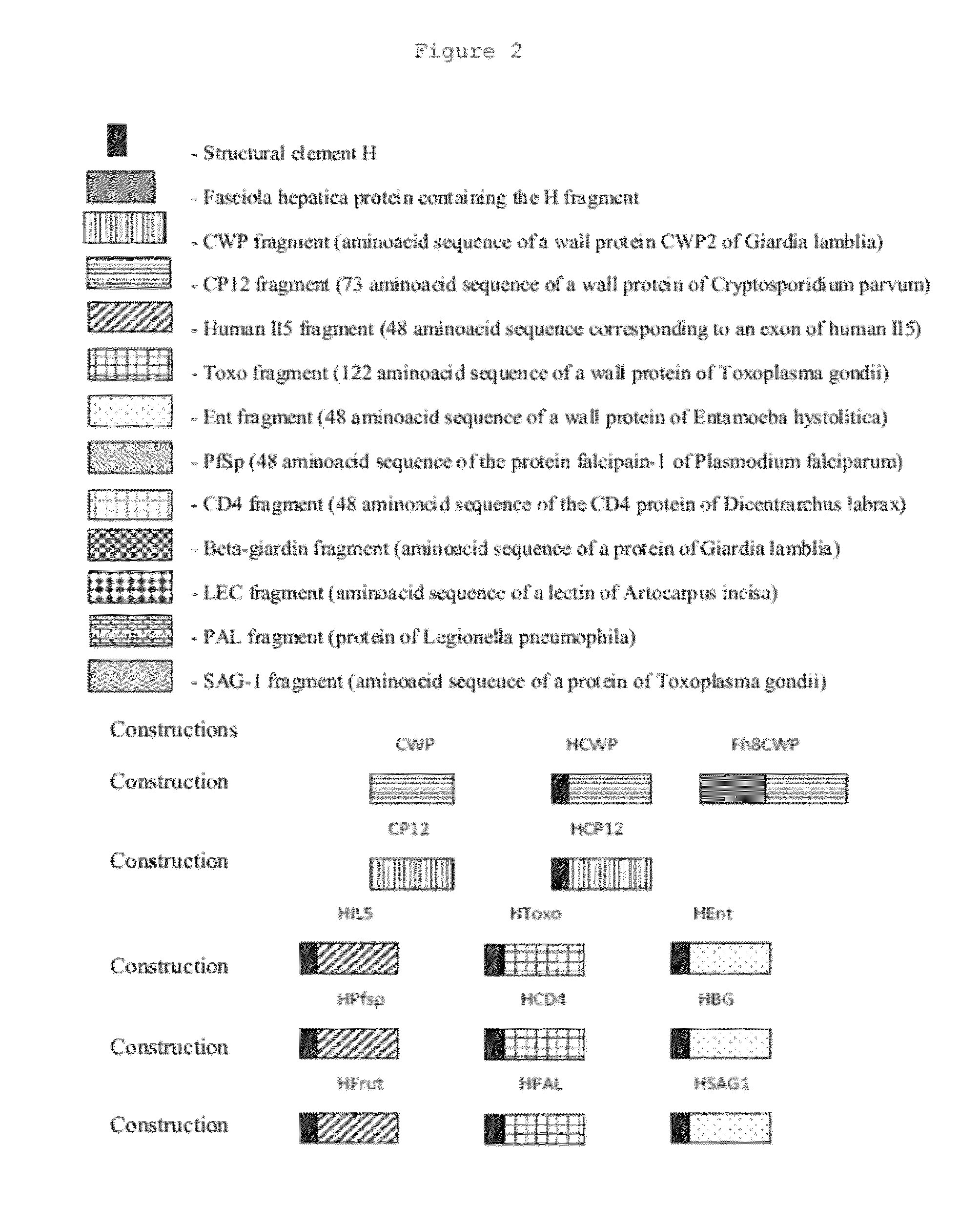
The invention relates to fusion proteins comprising an amino acid sequence of a fragment H corresponding to a fragment of a calcium binding protein excreted-secreted by adult worms of Fasciola hepatica, followed by an amino acid sequence corresponding to a unrelated protein or fragment of protein, pharmaceutical compositions, vaccines and adjuvants containing the immunogen, to a process for their preparation, another process for the production of antibodies and their use.The present invention relates to the preparation of immunogens by the addition of a peptide sequence.Thus the present invention is useful for producing an immune response, with increases in specific antibody titers in serum against proteins or other antigens and can be applied in particular for the production of specific polyclonal antibodies, immunotherapy and immunoprophylaxis. The addition of the polypeptide to a target antigen, either through the production of recombinant proteins containing the polypeptide or by addition or fusion of this polypeptide with the target antigen, induces a significant increase in the immunogenicity of these molecules, amplifying the immune response elicited by injection of this molecule in a subject susceptible to produce antibodies.
Owner:ESCOLA SUPERIOR AGRARIA DE COIMBRA +1
Pertussis PRN protein monoclonal antibody and application thereof
ActiveCN104892755AStrong specificityHigh potencyAntibacterial agentsImmunoglobulins against bacteriaBacteroidesPertussis vaccine
The invention provides a pertussis PRN protein monoclonal antibody and an application thereof, which belong to the biological product field. The monoclonal antibody is prepared by taking PRN protein in bordetella pertussis as immunogen immune mice, the obtained two monoclonal antibodies are respectively as, an amino acid sequence of a light-chain variable region is shown in a SEQ ID No.1, the amino acid sequence of a heavy-chain variable region is shown in a SEQ ID No.2, the amino acid sequence of the light-chain variable region is shown in a SEQ ID No. 3, and the amino acid sequence of the heavy-chain variable region is shown in a SEQ ID No.4. The provided monoclonal antibody enables no cross reaction with bordetella pertussis other protein and other antigen and pathogen, has the advantages of high specificity and high sensitivity for detecting the pertussis PRN components, can accurately detect PRN content of pertussis vaccine in a sample, and can be widely used in vaccine and clinical detection.
Owner:ABMAX BIOTECHNOLOGY CO LTD
Monoclonal antibody for hand-foot-mouth EV71 virus and application thereof
ActiveCN101812129BStrong specificityIncreased sensitivityImmunoglobulins against virusesTissue cultureProtein targetCarrier protein
The invention provides a monoclonal antibody for hand-foot-mouth EV71 virus, which is obtained through immunogen preparation by using VP1 protein of the EV71 virus as target protein to design and synthesize a polypeptide sequence and coupling the polypeptide sequence with vector protein serving as immunogen. The monoclonal antibody has no cross reaction with other proteins of the EV71 virus, CA16virus, other antigens or pathogens, has the advantages of high specificity and high sensitivity in detection, can accurately detect the level of the EV71 virus in a detected sample, and is expected to be widely applied in clinical detection.
Owner:ABMAX BIOTECHNOLOGY CO LTD
Recombinant porcine parvovirus antigenic protein and use thereof
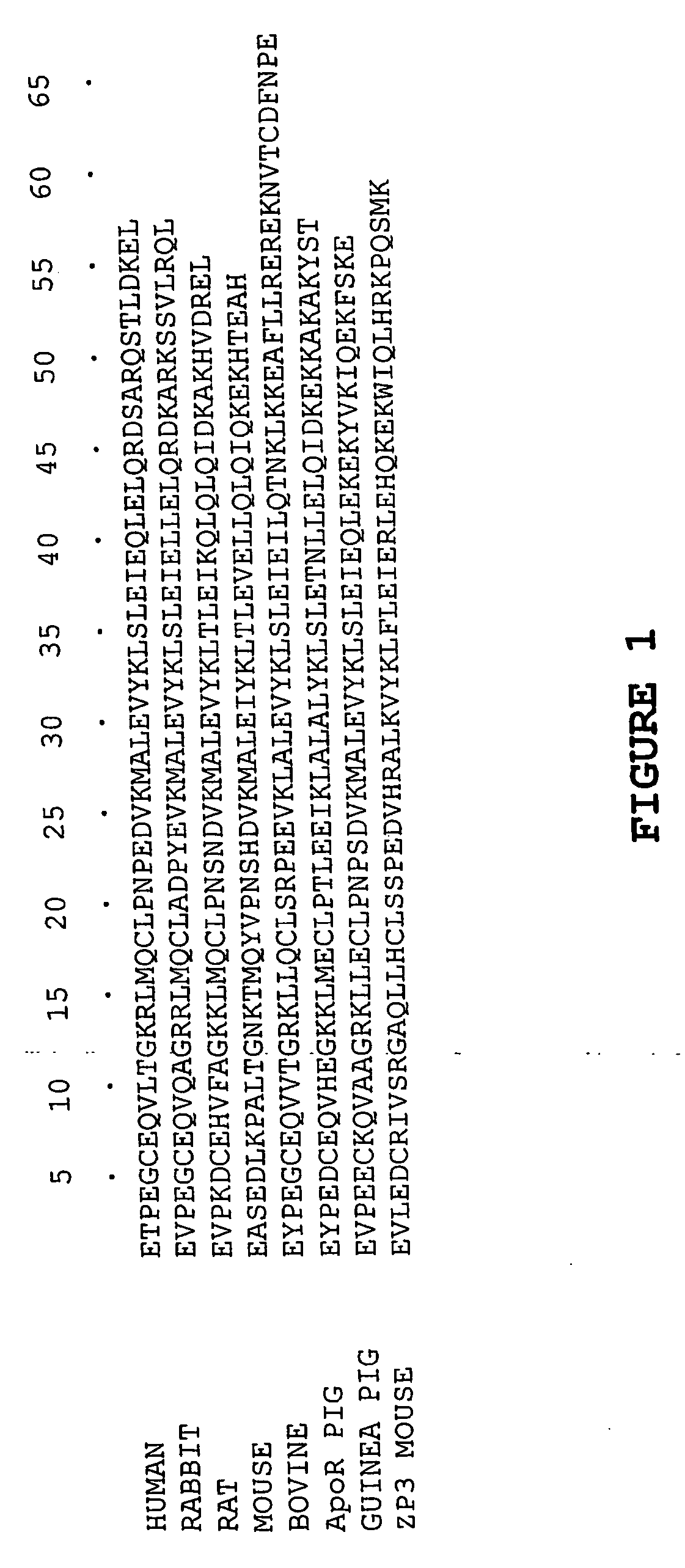
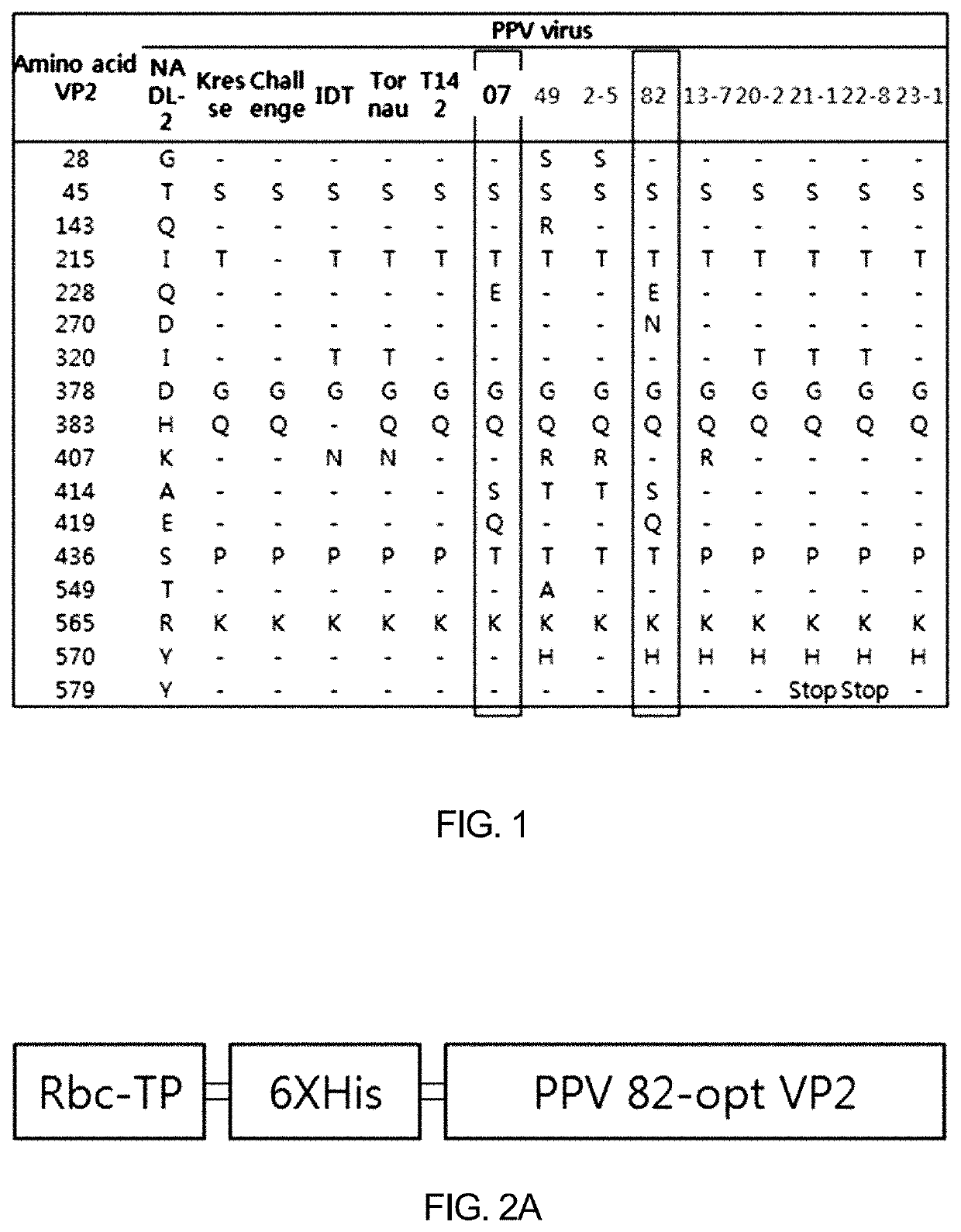
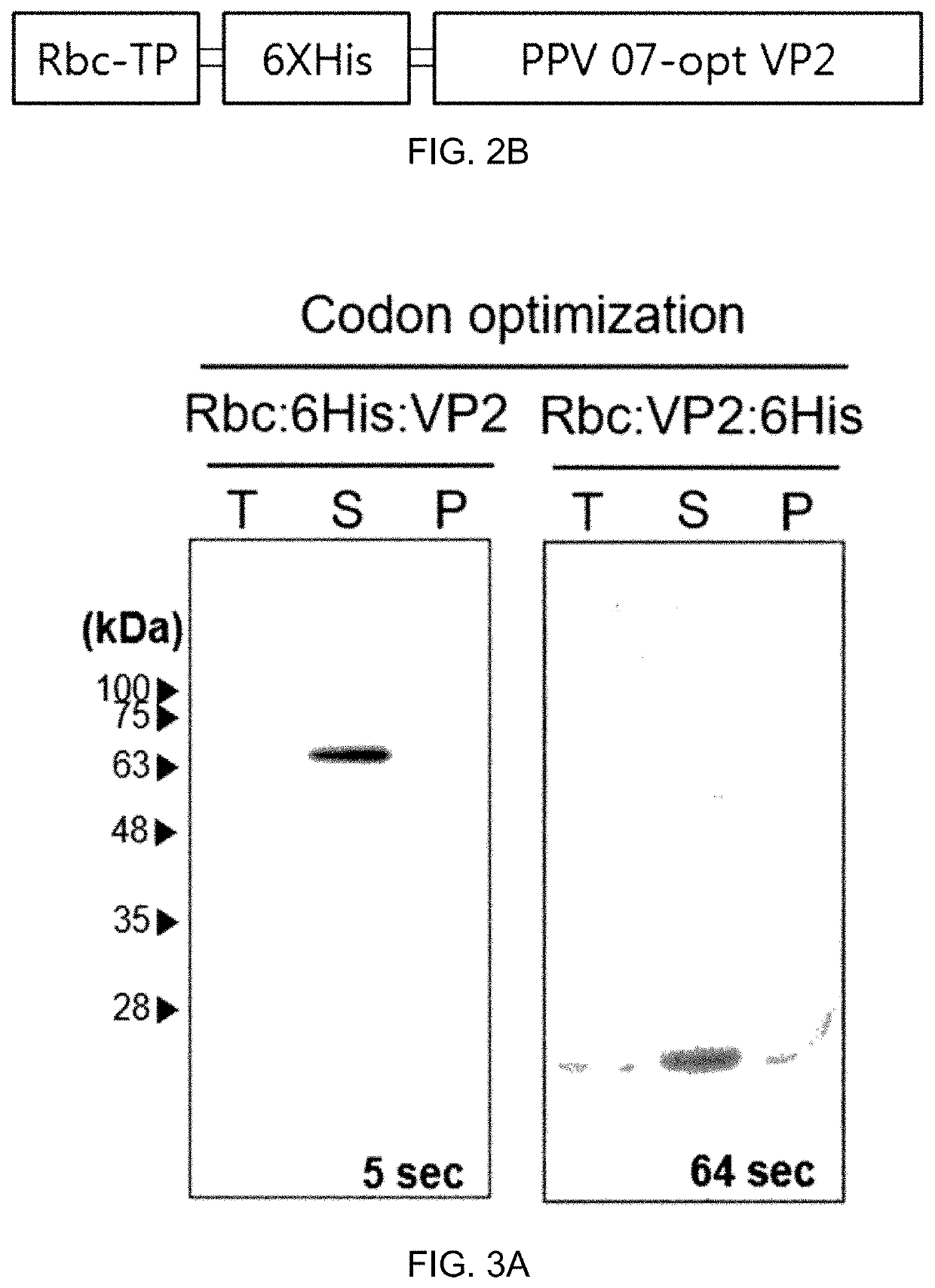
PendingUS20220016236A1Improve efficiencyImprove securityViral antigen ingredientsVirus peptidesPorcine parvovirus antigenHY Antigen
The present invention provides: a recombinant expression vector comprising a gene encoding a porcine parvovirus VP2 protein; a recombinant plant or a recombinant insect cell transformed with the vector; and a vaccine composition for a porcine parvovirus and a composition for diagnosing porcine parvovirus, both of which contain a porcine parvovirus VP2 protein obtained from the recombinant plant or the recombinant insect cell. When the recombinant plant or recombinant insect cell of the present invention is used, the porcine parvovirus antigenic protein can be produced with high efficiency, and the porcine parvovirus antigenic protein production method using the recombinant plant or recombinant insect cell has excellent safety and stability compared with other antigen production methods.
Owner:REPUBLIC OF KOREAANIMAL & PLANT QUARANTINE AGENCY +1
Poxvirus vectors encoding HIV antigens, and methods of use thereof
ActiveUS20200138938A1Improved cell surface expressionImproved expression stabilityViral antigen ingredientsAntibody mimetics/scaffoldsImmunopotencyHiv envelope
Poxvirus vectors encoding a synthetic HIV envelope antigen and other HIV antigens, as well as compositions containing such poxvirus vectors and uses of such poxvirus vectors as vaccines to provide improved immunity against HIV, are provided. Also provided are vaccine combinations containing the disclosed poxvirus vectors, adenovirus vectors encoding one or more HIV antigens, and one or more isolated HIV antigenic polypeptides, and methods of using the vaccine combinations to provide improved immunity against HIV.
Owner:JANSSEN VACCINES & PREVENTION BV +1
Antigen polypeptide for inducing liver cancer specific cytotoxic T lymphocytes and application of antigen polypeptide
ActiveCN111944018AIncrease positive rateStrong positive rateCompound screeningApoptosis detectionT lymphocyteImmunogenicity
The invention discloses an antigen polypeptide for inducing liver cancer specific cytotoxic T lymphocytes and application of the antigen polypeptide. The amino acid sequence of the antigen polypeptidefor inducing liver cancer specific cytotoxic T lymphocytes comprises a sequence as shown in Seq ID No. 1. The antigen polypeptide provided by the invention can specifically and efficiently induce liver cancer cytotoxic T lymphocytes, and the liver cancer specific cytotoxic T lymphocytes generated by induction have better performance in immunogenicity, positive rates of antigen-loaded cytotoxic Tlymphocyte cells and killing efficiency in in-vitro cell experiments, compared with CTL induced by other existing antigen polypeptides. The antigen polypeptide is adopted to induce cytotoxic T lymphocytes capable of efficiently and specifically killing liver cancer tumor cells, the preparation method is efficient and convenient, compared with other immune cell therapies, the antigen polypeptide has the characteristics of low cost, high yield and simple technological process, and a new scheme and way are provided for clinical application of liver cancer immune cell therapy.
Owner:深圳市乐土生物医药有限公司
Tolerizing agents
InactiveUS7910113B2Facilitates systemic and mucosal tolerance inductionRestoring immunogenicityNervous disorderAntibody mimetics/scaffoldsAntigenTolerability
Described herein is the development of fusion proteins useful for inducing tolerance in a subject. In particular embodiments, the tolerizing agents are useful for influence autoimmune, inflammatory, and / or allergic reactions. Example tolerizing fusion proteins contain a targeting portion (which delivers the fusion protein) and a toleragen or allergen or other antigen to which tolerance is desired in a subject. In particular examples, it is demonstrated that a pσ1 fusion protein, when administered orally, facilitates systemic and mucosal tolerance. Also described is the nasal delivery of fusion proteins, for instance for restoring immunogenicity.
Owner:UAB RES FOUND +1
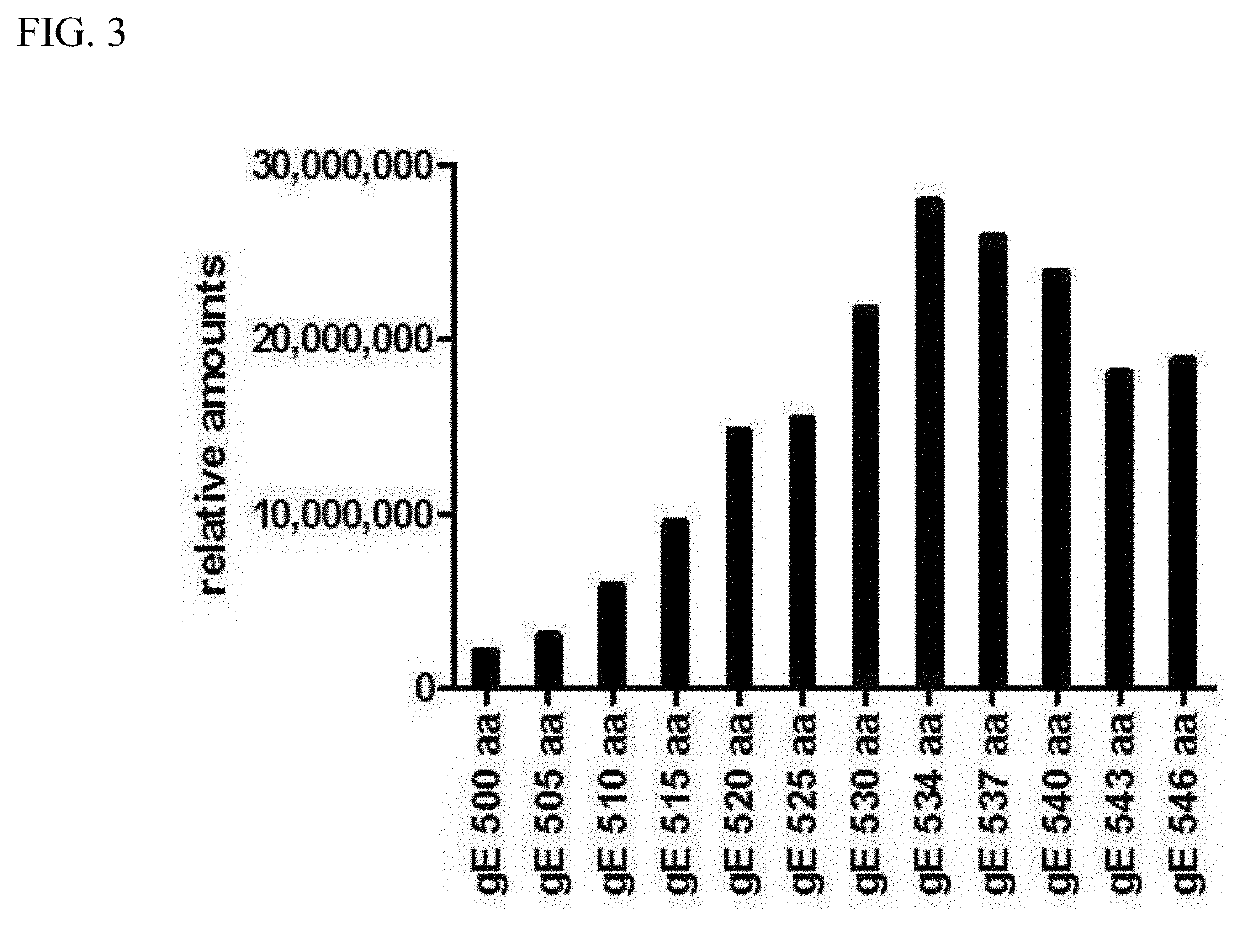
Antigen variant of varicella zoster virus and use thereof
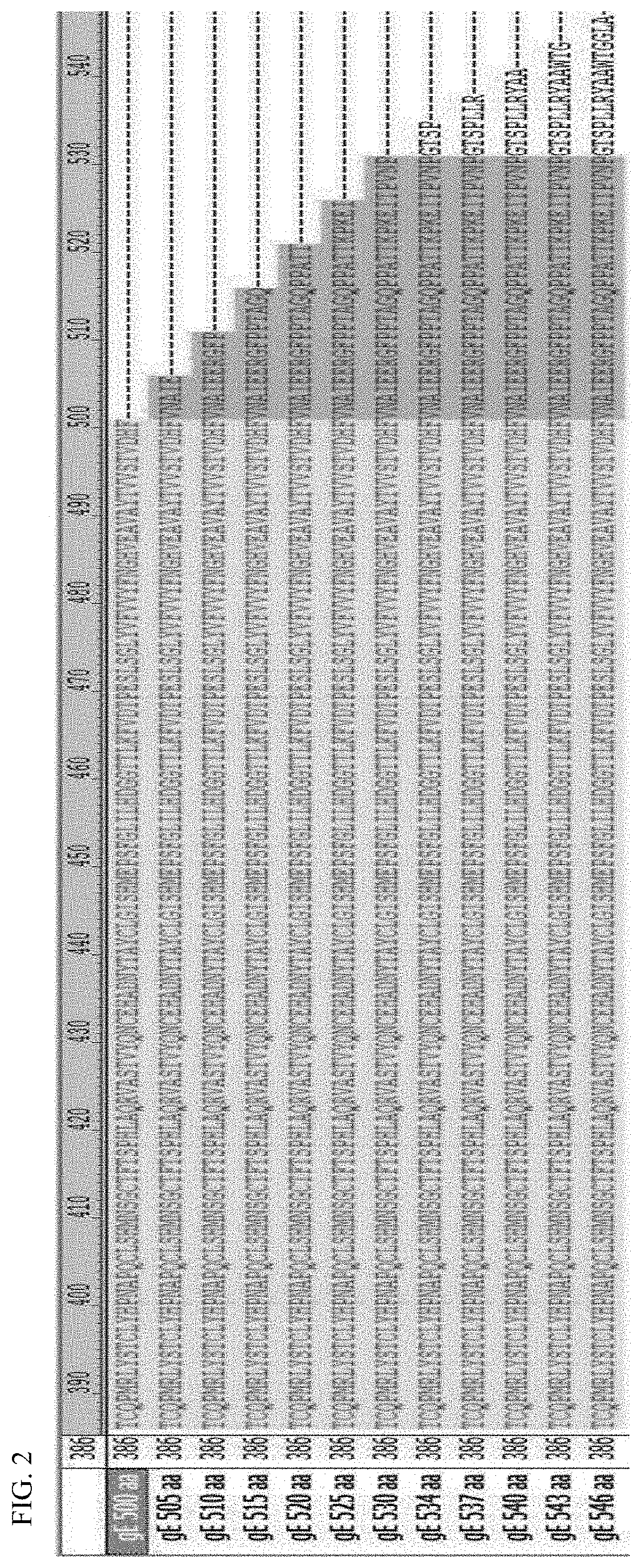
ActiveUS20210187099A1High expressionImproving immunogenicityViral antigen ingredientsVirus peptidesChickenpoxVaricella-zoster virus antigen
An antigen variant and a use thereof are disclosed. The antigen variant is a protein, among surface proteins (gE) of the varicella zoster virus, exhibits a high expression level and high immunogenicity, and thus, when the antigen variant is used as a vaccine composition, the vaccine composition has more excellent safety compared to a live virus vaccine, and the antigen variant exhibits a higher expression level in a host cell compared to other antigens. The antigen variant is useful as a vaccine for preventing or treating chicken pox or herpes zoster caused by the varicella zoster virus.
Owner:MOGAM INST FOR BIOMEDICAL RES
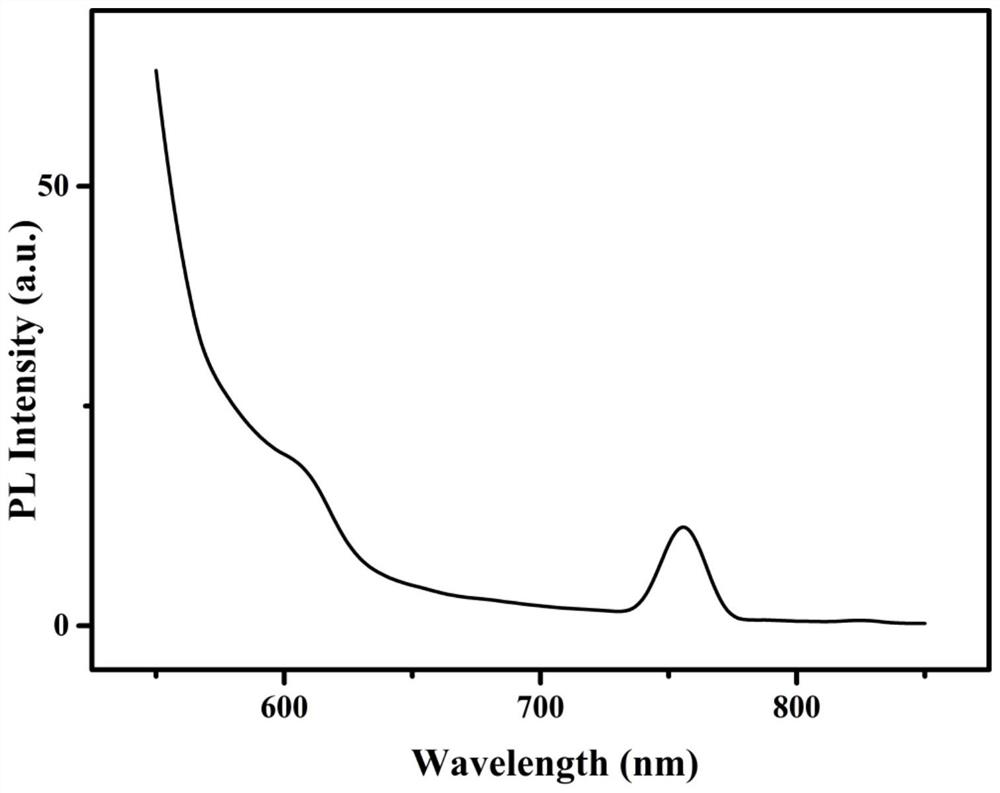
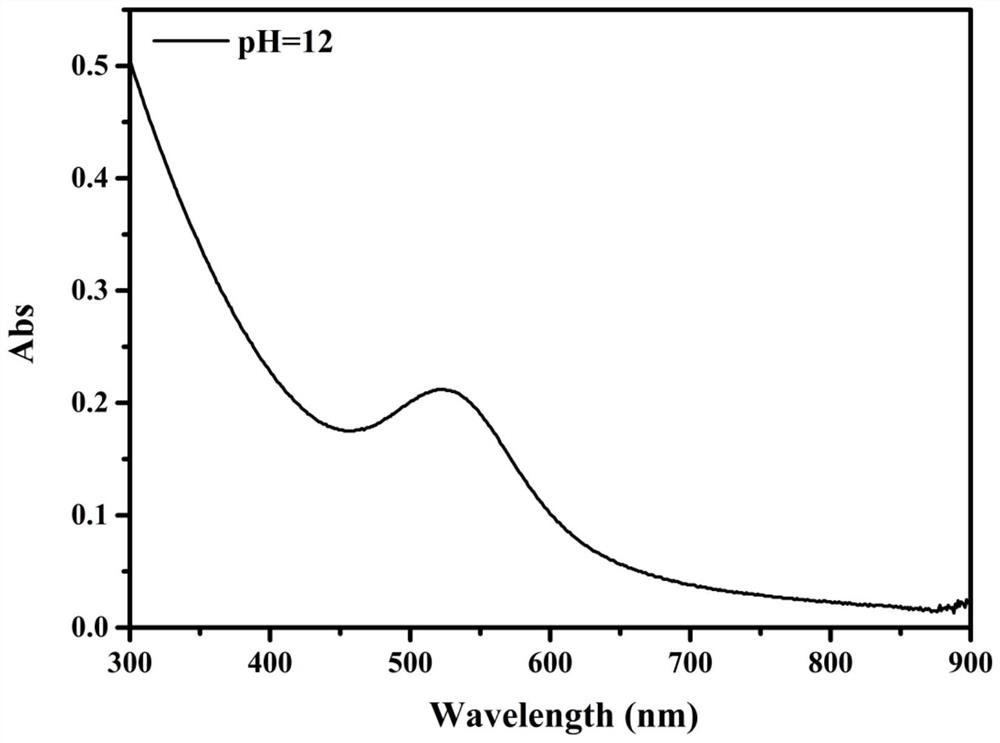
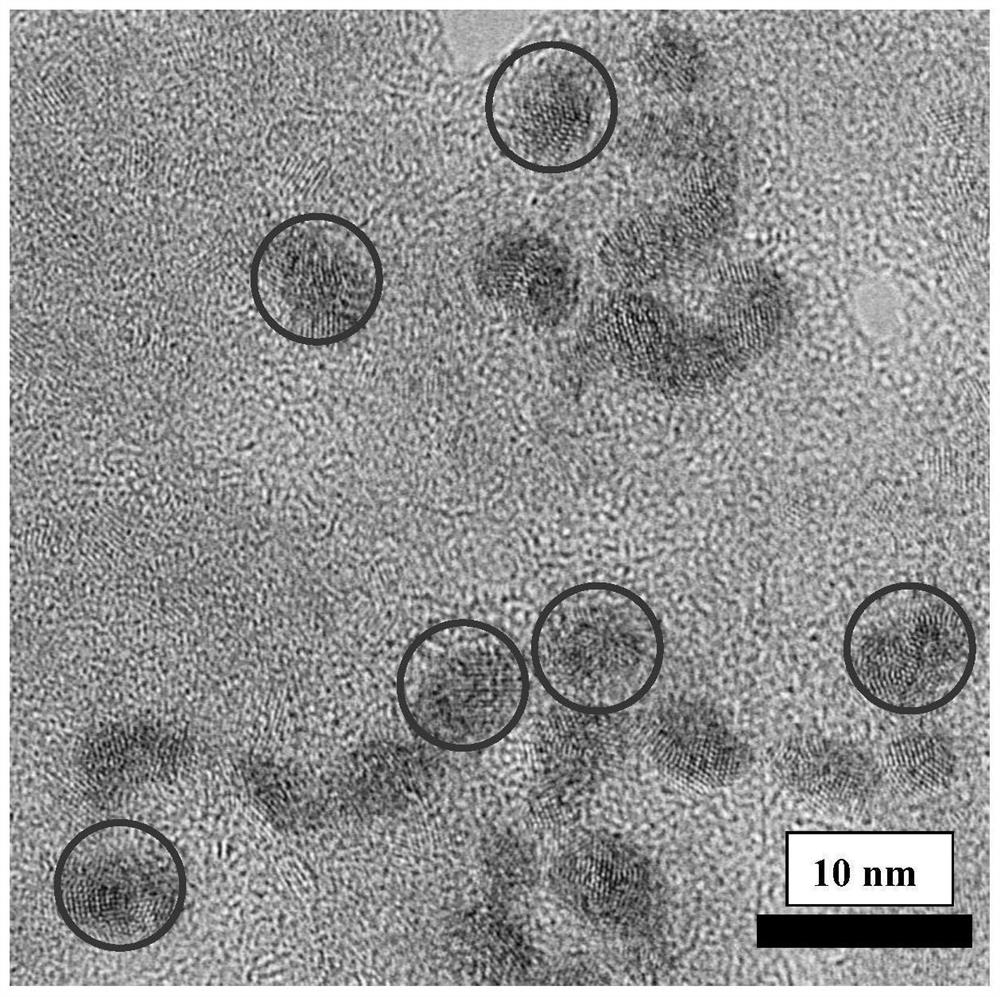
Preparation method of a spectral near-infrared electrochemiluminescence immunosensor
ActiveCN112147132BNo distractionHigh selectivityChemiluminescene/bioluminescenceMaterial electrochemical variablesElectrochemiluminescenceHY Antigen
The invention belongs to the field of analytical techniques and methods, and relates to the preparation of a spectral type, near-infrared electrochemiluminescence (~915nm) immunosensor, which mainly includes (1) using methionine as a stabilizer to prepare a water-soluble Au‑Ag bimetallic nanoclusters, (2) gold-silver bimetallic nanoclusters labeled secondary antibody (Au‑Ag|Ab 2 ), (3) preparation of spectral near-infrared electrochemiluminescence sensor with gold-silver bimetallic nano-gold-silver clusters as markers based on the principle of sandwich immunity, and (4) drawing of the working curve. The detection sensitivity of the prepared spectral electrochemiluminescence immunosensor is high, and other antigenic proteins do not interfere with the sensing and detection of the target antigen of the invention.
Owner:SHANDONG UNIV
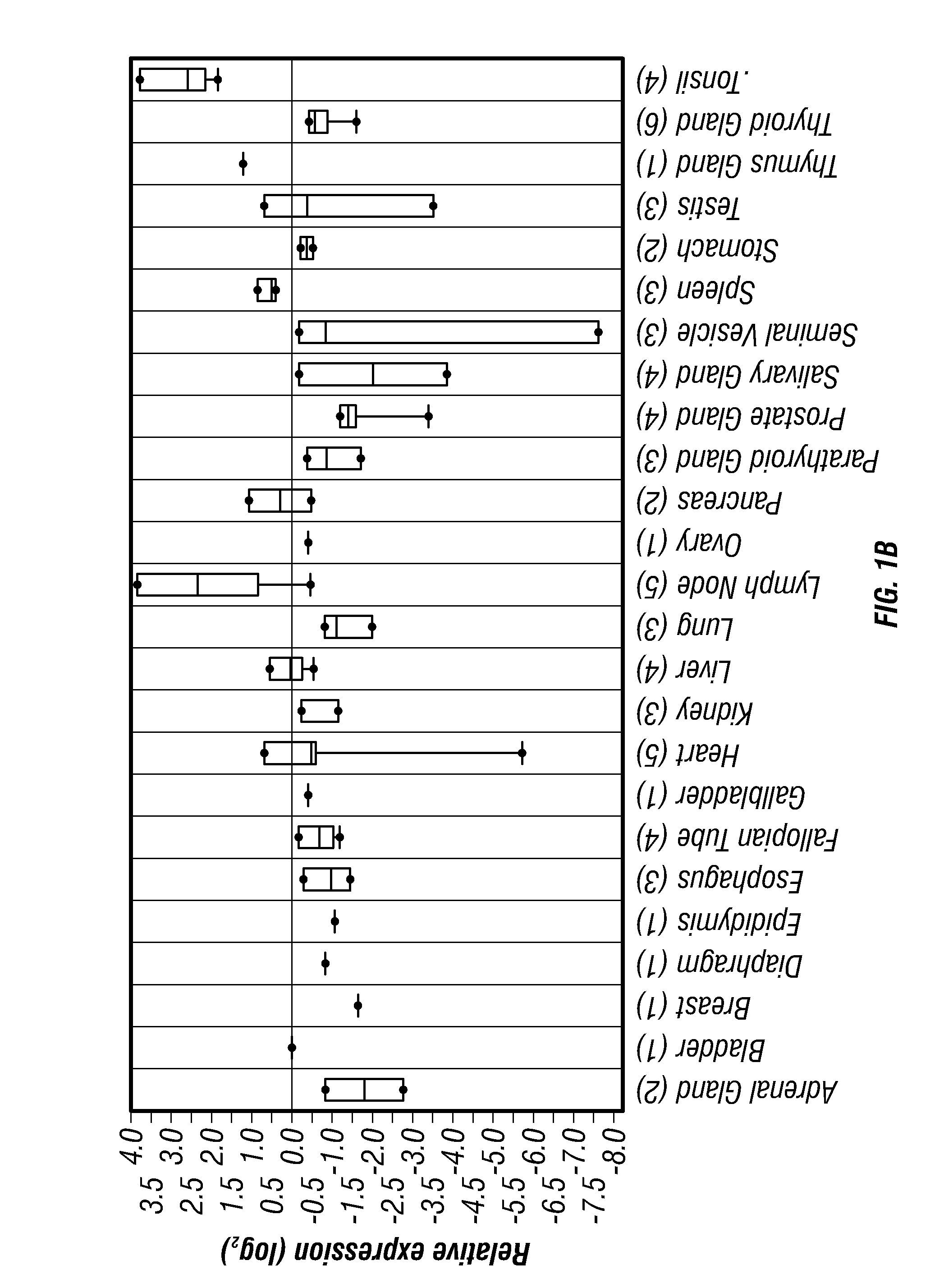
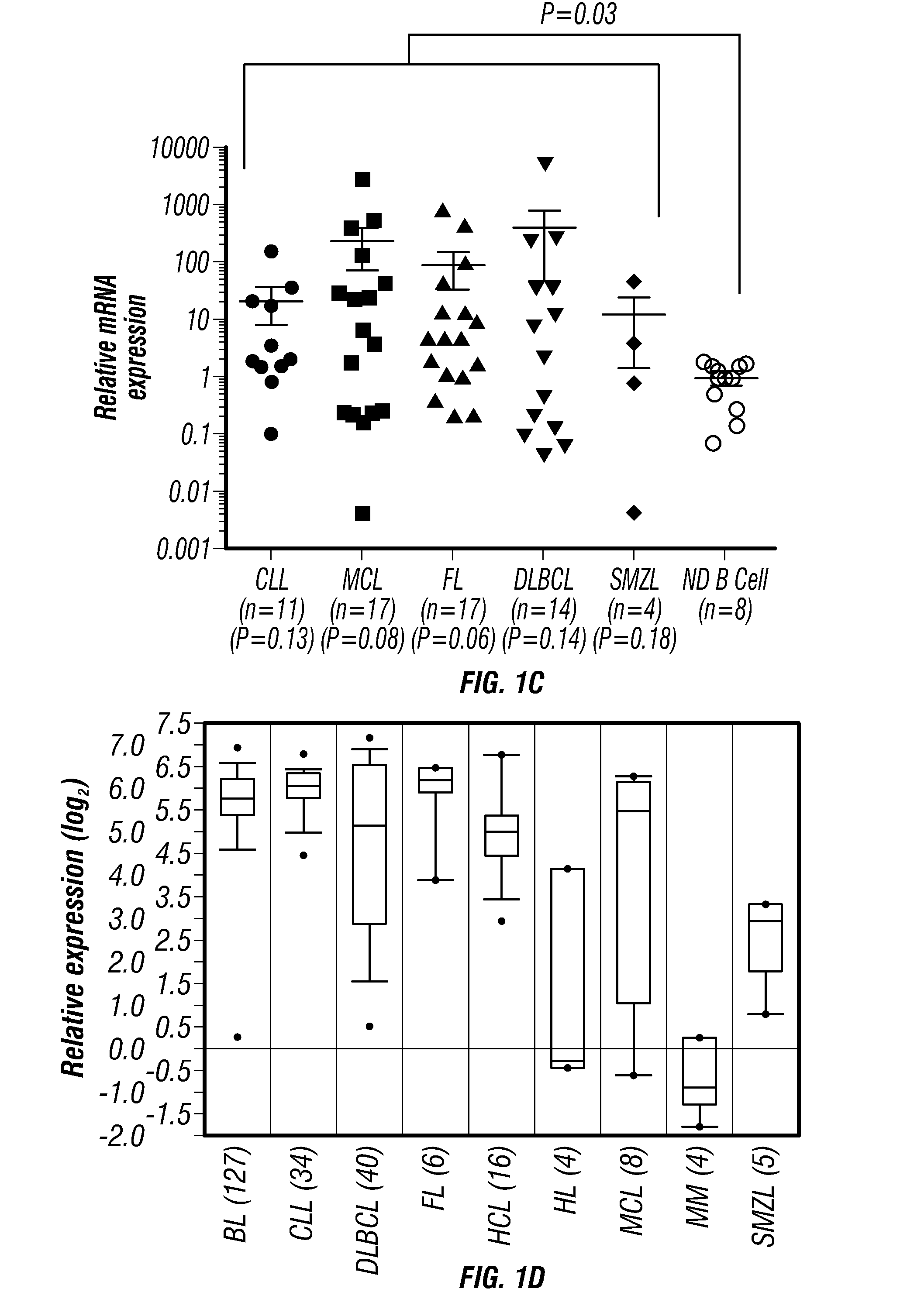
Tcl1 peptides for immunotherapy
Provided are TCL1 peptides that bind to MHC I (HLA-A2) on tumor cells or other antigen-presenting cells and are recognized by T-cell receptors on T cells. The TCL1 peptides may be therapeutically used to treat a cancer, such as a B cell malignancy, leukemia, or lymphoma. Methods for expanding a population of T cells that target TCL1 are also provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com