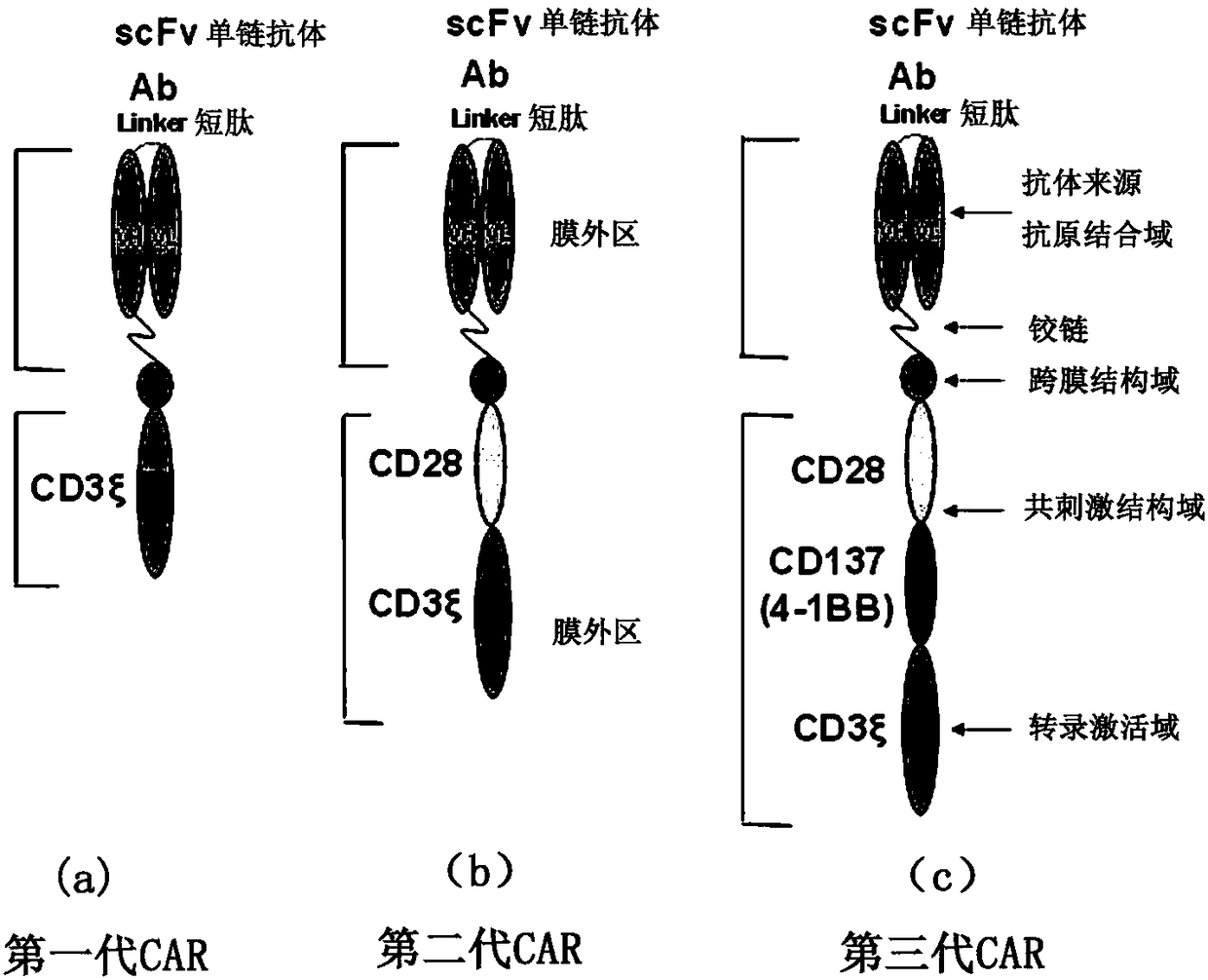
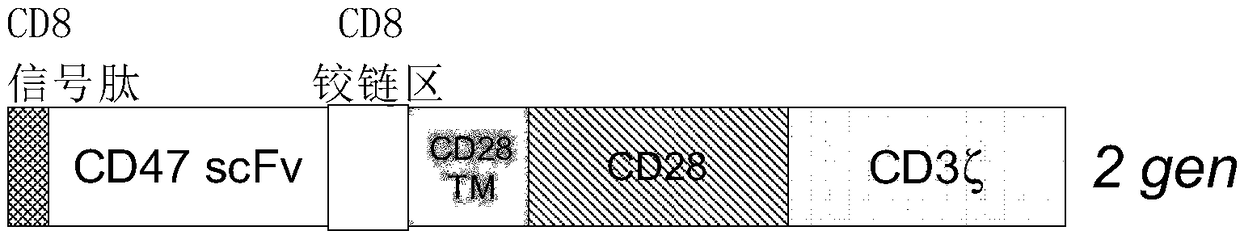
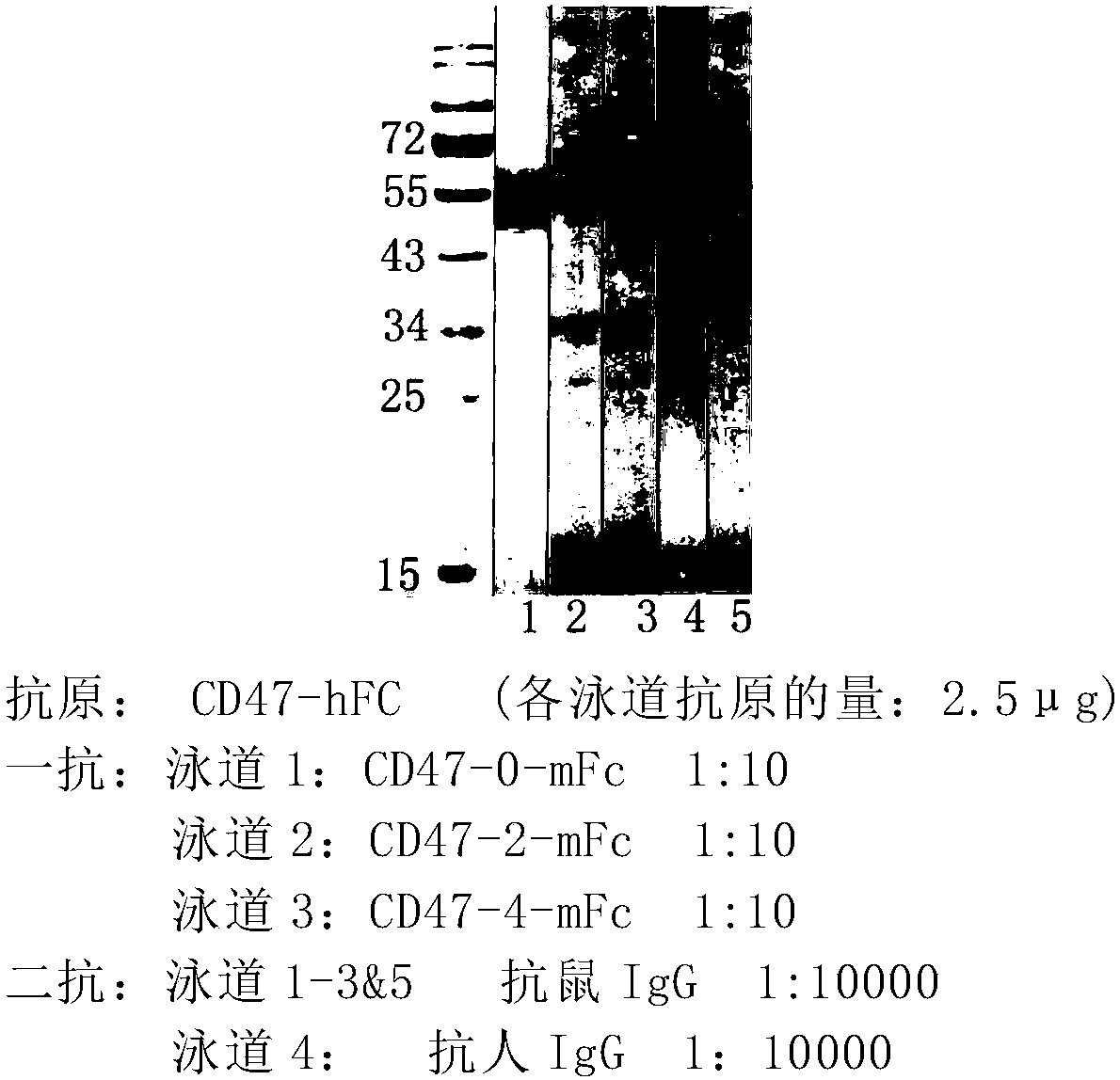
Cd47-car-t cells
A host cell and fusion protein technology, applied in the field of adoptive immune gene therapy of tumors, can solve the problems of high recurrence rate and low safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148] Example 1 Production of CAR lentivirus
[0149] Lentiviruses were prepared by the following steps:
[0150] Day 1:
[0151] 1. Place 5×10 6 HEK293FT cells were seeded into 100 mm diameter petri dishes;
[0152] Day 2:
[0153] 2. Check to make sure the cells are 70%-90% confluent;
[0154] 3. Prepare the transfection complex for each 100 mm diameter Petri dish as follows:
[0155] a. In 1.5ml tube A: dilute 2.5μg CAR (chimeric antigen receptor) DNA plasmid (plasmid) and 20μL lentiviral packaging mix (ALSTEM, catalog number VP100; see Appendix B3) to 0.5ml DMEM or Opti -MEM serum-free medium, mix gently;
[0156] b. In 1.5ml tube B: Dilute 30μL Nanofect Transfection Reagent (ALSTEM, catalog number NF100) into 0.5ml DMEM or Opti-MEM serum-free medium, mix gently;
[0157] c. Add the NanoFect / DMEM in tube B to the DNA / DMEM solution (tube A), vortex for 5-10 seconds, and incubate the DMEM-plasmid-NanoFect mixture at room temperature for 15 minutes;
[0158] 4. Add a...
Embodiment 2
[0169] Example 2 lentiviral packaging system
[0170] Product Description
[0171] Product name: SuperLenti TM Lentivirus Packaging System
[0172] manual:
[0173] For the production of lentiviral particles, three components are generally required: 1) a lentiviral vector containing the foreign gene of interest, 2) a packaging vector containing all the necessary viral structural proteins, 3) expressing vesicular stomatitis virus (VSV) sugars Envelope carrier for protein (G). The third-generation lentiviral packaging system provides maximum biosafety because the lentiviral Rev gene is provided as a separate vector from other structural genes, further eliminating the possibility of reverse recombination of the vector into replication-competent virus particles. Third-generation lentiviral packaging mixes only support lentiviral expression vectors with chimeric 5’LTRs, where the HIV promoter is replaced by CMV or RSV, thus making it independent of TAT.
[0174] The SuperLent...
Embodiment 3
[0187] Example 3 Isolation of Peripheral Blood Mononuclear Cells (PBMC) in Whole Blood
[0188] Whole blood (Stanford University Hospital Blood Center, Stanford, CA) was collected from a single individual or multiple individuals (depending on the volume of blood required) and placed in 10 mL Heparin vacutainers (Becton Dickinson Company).
[0189] NOTE: Blood should be processed within two hours of blood collection to ensure maximum cell yield. Blood can be stored overnight at room temperature (indoors) for processing the next day; however, there will be some loss in cell yield. Blood should not be stored in empty tubes at 4°C. In a 50ml conical centrifuge tube, mix approximately 10ml of anticoagulated whole blood with sterile phosphate buffered saline (PBS) for a total volume of 20ml (PBS, pH 7 / 4, Ca-free 2+ / Mg 2+ ).
[0190] In a separate, sterile 50 mL conical centrifuge tube, pipette into 15 mL of Ficoll-Paque PLUS (GE Healthcare, 17-1440-03). Very gently layer 20ml ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com