Dendritic cell tumor vaccine and its preparation and use
A technology of dendritic cells and cells, applied in the fields of biology and medicine, can solve the problems of unfavorable applications, limited clinical use, low transfection efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
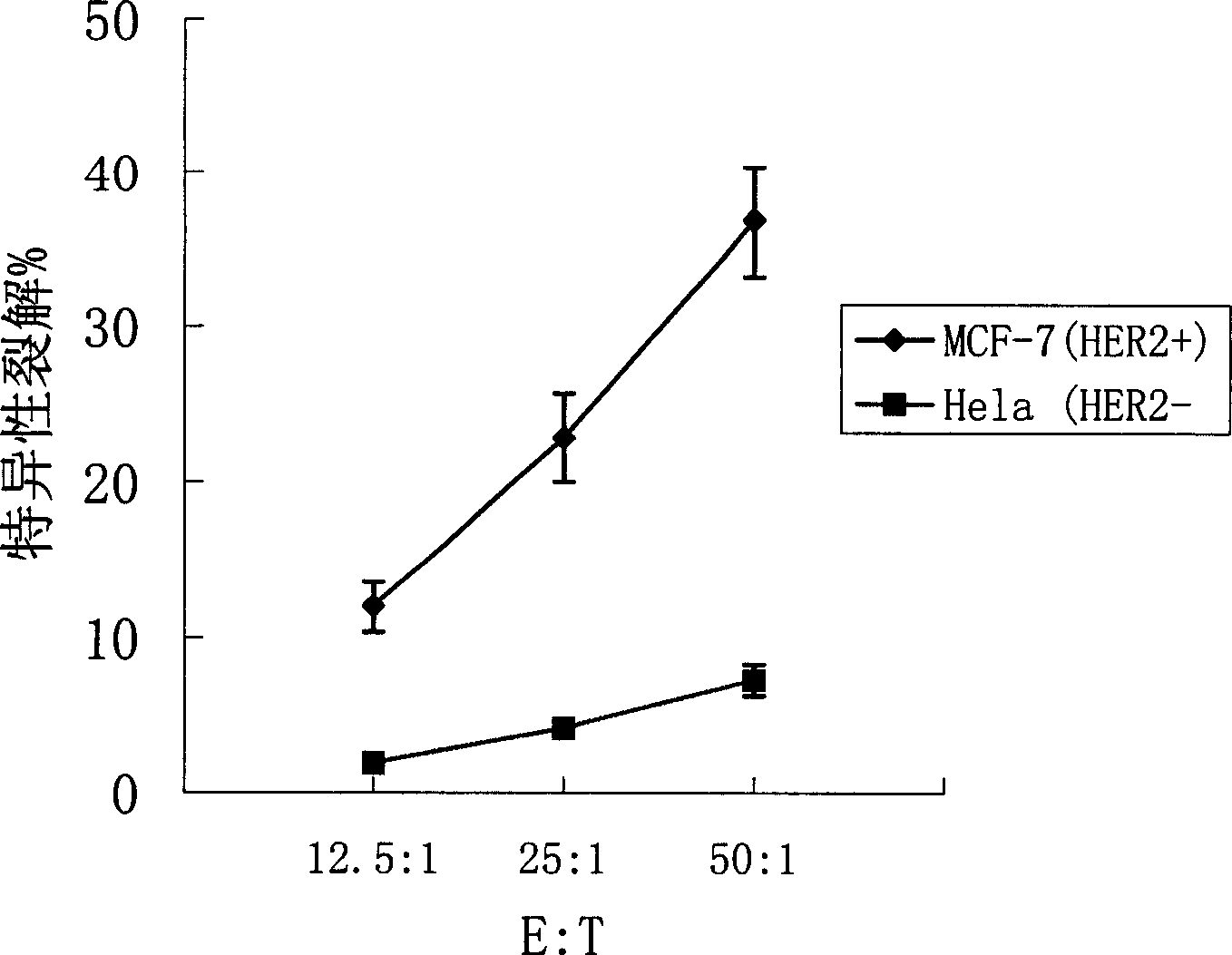
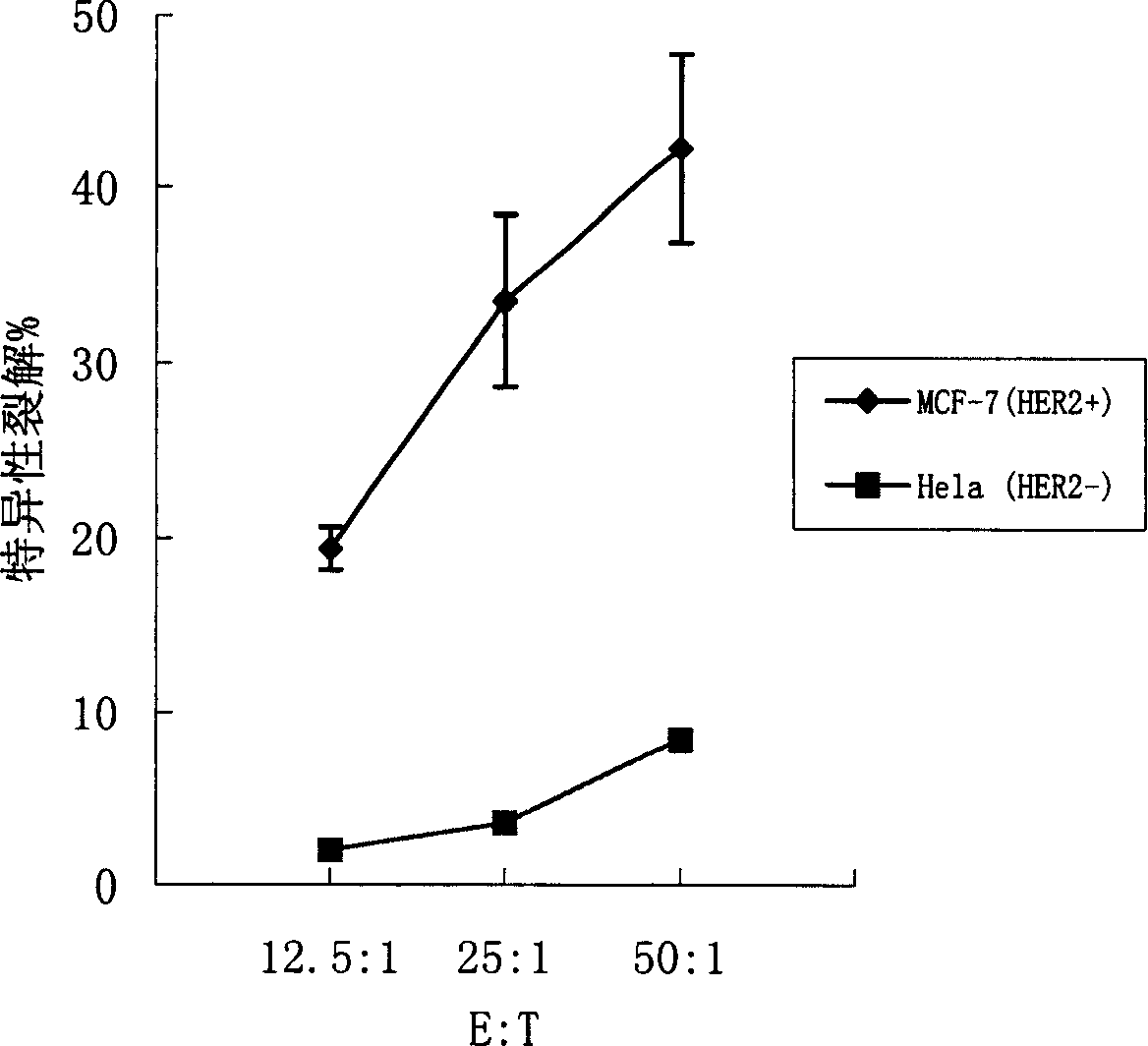
[0097] Establishment and screening of tumor cell lines with high expression of HER2
[0098] The present invention uses the following primers
[0099] Upstream primer: 5'CGCTCGAGCACC ATG GAGCTGGCGGC 3' (SEQ ID NO: 1)
[0100] Downstream primer: 5'CGAAGCTTGAGCAGAGAGCCAGCCC 3' (SEQ ID NO: 2)
[0101] The human HER2 proto-oncogene extracellular region fragment was cloned from the human breast cancer cell line SK-BR-3 (available from the American Type Culture Collection, ATCC HTB-30) by standard RT-PC method, and the sequence was correct. Insert pCDNA3.1 (Invitrogen) to construct HER2 eukaryotic expression vector pCDNA3.1-HER2. Using conventional liposome transfection method, the expression vector pCDNA3.1-HER2 was introduced into human breast cancer cell line MCF-7 (available from American Type Culture Collection, ATCC HTB-22) and mouse colon cancer cells. Line CT26 (can be purchased from the American Type Culture Collection, ATCC CRL-26), was selected and cultivated by G418...
Embodiment 2
[0103] Preparation and purification of HER2 mRNA
[0104] (a) Preparation of HER2 mRNA:
[0105] For the tumor cell clones highly expressing HER2 obtained in Example 1 (ie breast cancer cell line MCF-7 or mouse colon cancer cell line CT26 highly expressing HER2), a large amount of amplification was performed, and then the cells were collected. Wash twice with PBS, discard the supernatant, and place in an ice bath. Add guanidine isothiocyanate denaturing solution (20ml / 1×10 8 Cells), mix quickly to lyse the cells quickly and thoroughly. Add 2ml of 2mol sodium acetate (PH 4.0), mix well and transfer to a 50ml centrifuge tube. Add 22ml of phenol: chloroform: isoamyl alcohol (25:24:1), mix vigorously by inverting, and place on ice for 10 min. Carefully pipette the upper aqueous phase containing RNA, transfer to a new 50ml centrifuge tube, add an equal volume of isoamyl alcohol, and precipitate at -20°C for 1 hour. Centrifuge at 4°C, 12000rpm×15min. Discard the supernatant, a...
Embodiment 3
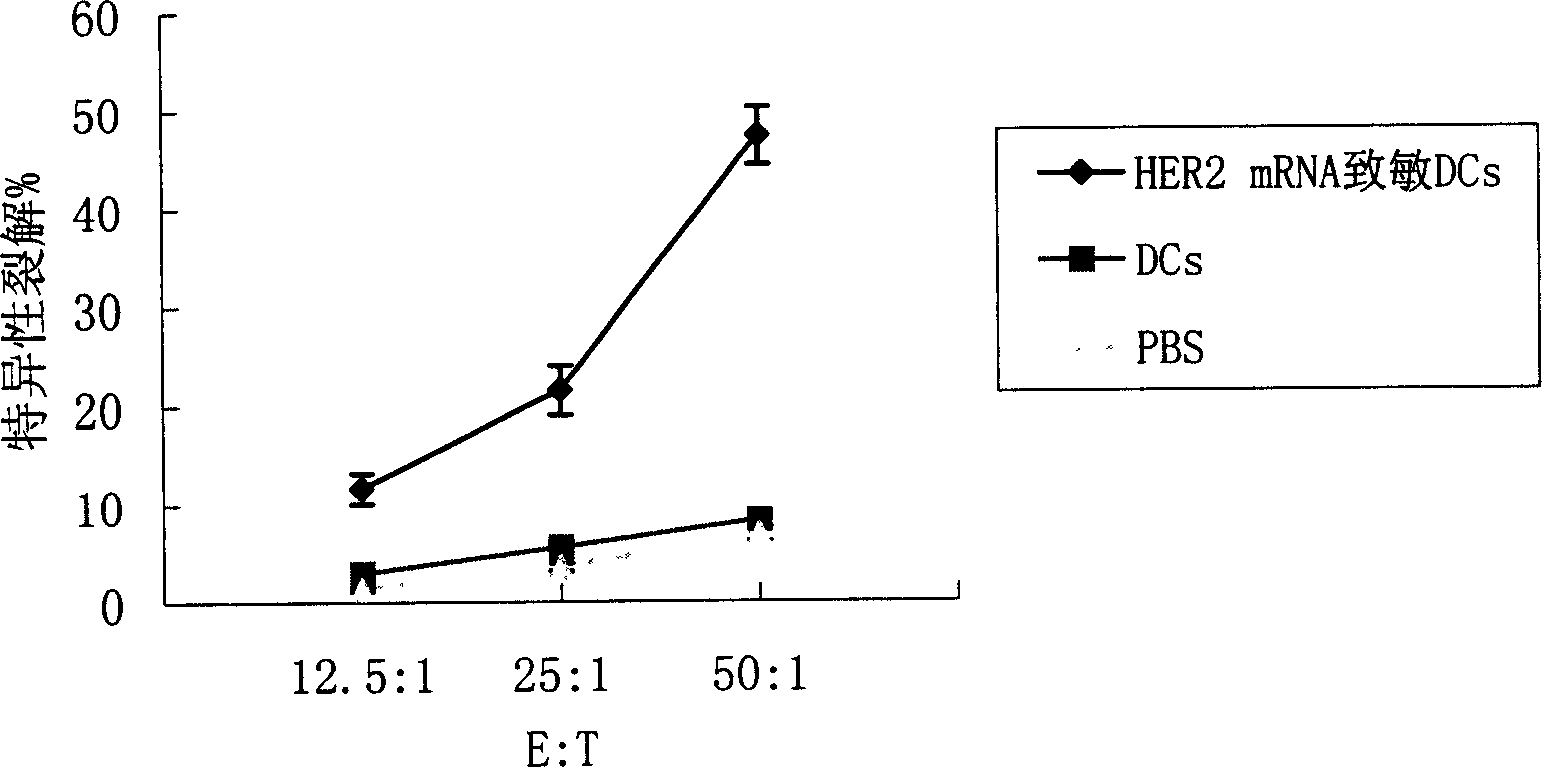
[0109] HER2 mRNA sensitized dendritic cells
[0110] Collect the human or mouse cultured dendritic cells cultured to day 6, wash twice with serum-free RPMI 1640, and adjust the cell concentration to 1×10 with serum-free RPMI 1640 7 / ml. Take 0.4ml of cell suspension and add it into a 0.2-cm electric shock cup, add 30μg of HER2 mRNA at the same time, and mix well. After 10 minutes of ice-bath in the electric shock cup, wipe it dry, and put it into the electric shock chamber of the ECM 830 electroporator of BTX Company for electroporation. After electroporation, gently take out the electric shock cup, and ice bath for 10 minutes. Carefully remove the cell suspension and add fresh DC complete medium at 37 °C 5% CO 2 Continue to culture in the incubator for 20 hours, add TNF-α to stimulate for 24 hours to promote DC maturation, collect cells, and irradiate with 30 Gy radiation for later use. At the same time, dendritic cells were transfected with EGFP mRNA and detected by flow ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com