Tumor antigenic polypeptide and application thereof as tumor vaccine
An antigen and vaccine technology, which can be used in anti-tumor drugs, medical preparations containing active ingredients, peptide sources, etc., and can solve problems such as strength differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
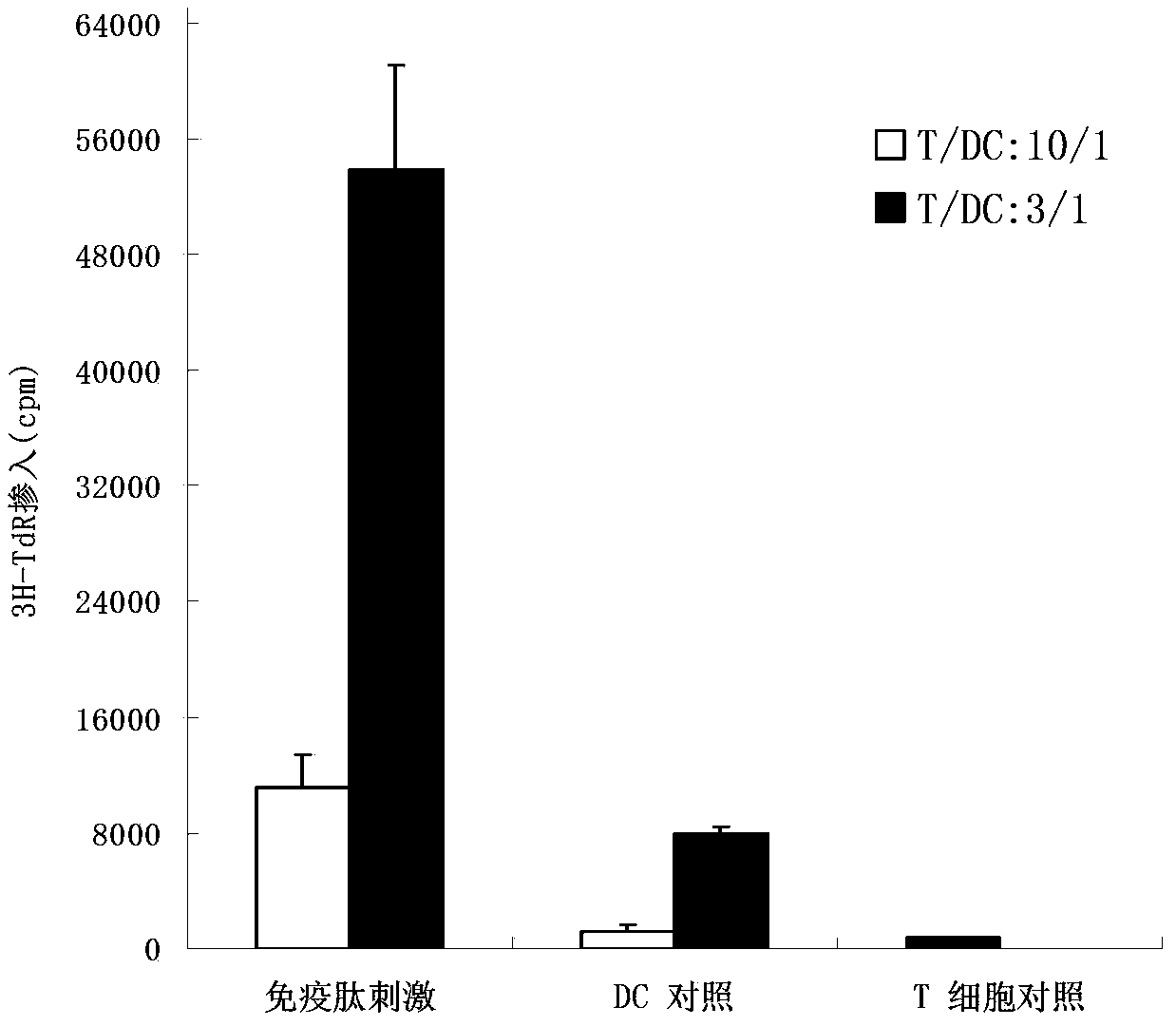
Image
Examples
Embodiment 1
[0073] The synthesis of embodiment 1 antigenic peptide
[0074] The synthetic polypeptide MTPGTQSPFFLLLLLLTVLTVVTGS (SEQ ID NO: 1), hereinafter referred to as antigenic peptide. The synthesized peptide was purified to 97%. Lyophilized antigenic peptides were dissolved in dimethyl sulfoxide (25 mM) and stored at -80°C.
[0075] The same method was used to synthesize polypeptides FLLLLLTVLT (SEQ ID NO: 2), LLLLLTVLTV (SEQ ID NO: 3), LLLLTVLTVV (SEQ ID NO: 4), FFLLLLLTVL (SEQ ID NO: 5), GTQSPFFLLL (SEQ ID NO: 6), TQSPFFLLLL (SEQ ID NO: 7), respectively referred to as antigenic peptide 1, antigenic peptide 2, antigenic peptide 3, antigenic peptide 4, antigenic peptide 5 and antigenic peptide 6.
Embodiment 2
[0076] Example 2 Preparation of dendritic cells (Dendritic Cells, DCs)
[0077] Mononuclear cells (PBMC) in peripheral blood include lymphocytes and monocytes, and their volume and shape specific gravity are about 1.075. Use the Ficoll-Hypaque separation medium with a specific gravity of 1.077 for density gradient centrifugation, so that cells with a certain specific gravity are distributed according to the corresponding density gradient, and various blood cells can be separated.
[0078]Obtain the white blood cell concentrate of healthy volunteers from the Hong Kong Red Cross Blood Center, dilute it with PBS to an appropriate multiple, and then add it to the surface of the Ficoll-Hypaque separation medium, centrifuge at 800g density gradient for 30min at 18-20°C, and after centrifugation is completed, the centrifuge tube The content is divided into three layers, the upper layer is plasma (containing platelets), the middle layer is separation fluid, and the bottom layer is red...
Embodiment 3
[0079] Preparation of embodiment 3 T cells (nylon cotton method)
[0080] T cells have short and few villi on the surface, while B cells have many and long villi. Due to the different smoothness of the cell surface, B cells are easy to attach to nylon cotton fibers at 37°C, while T cells do not have this ability. Using this property, T cells and B cells can be separated.
[0081] Preparation of nylon cotton column: After sterilizing 1g of nylon cotton / column, add RPMI1640 medium and place it at 37°C for 2 hours to allow RPMI1640 to flow out. Resuspend the above non-adherent cells in an appropriate volume of RPMI1640 so that the cell concentration is 0.5-1.0x10 8 / ml, then added to the prepared nylon cotton column, 2ml / column, incubated at 37°C for 1 hour, added RPMI1640 to the nylon cotton column, 10ml / column, eluted the cells not adsorbed on the nylon cotton, collected and eluted The solution is the T cell suspension. Centrifuge at 400g for 5min, resuspend the cell pellet ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com