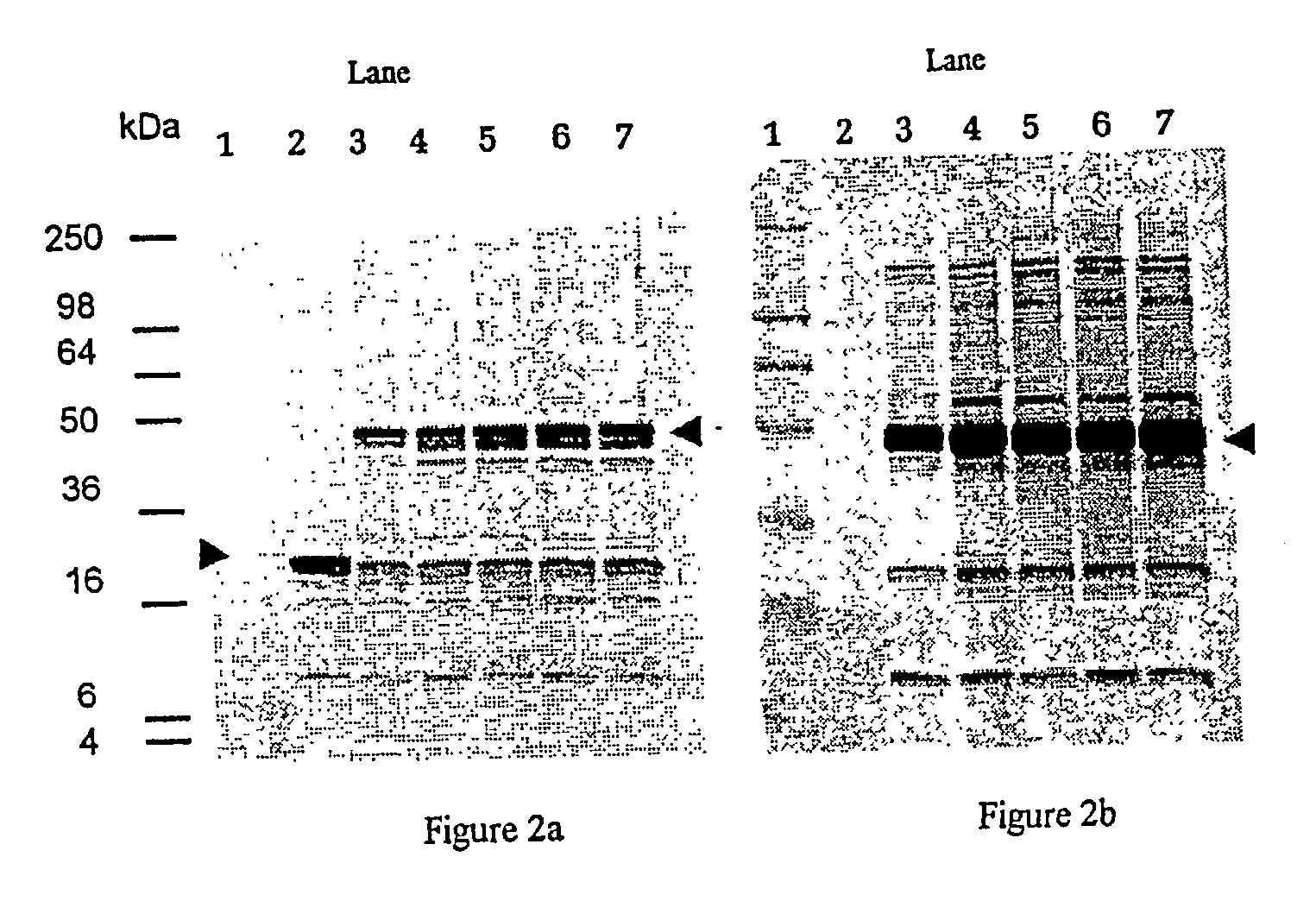
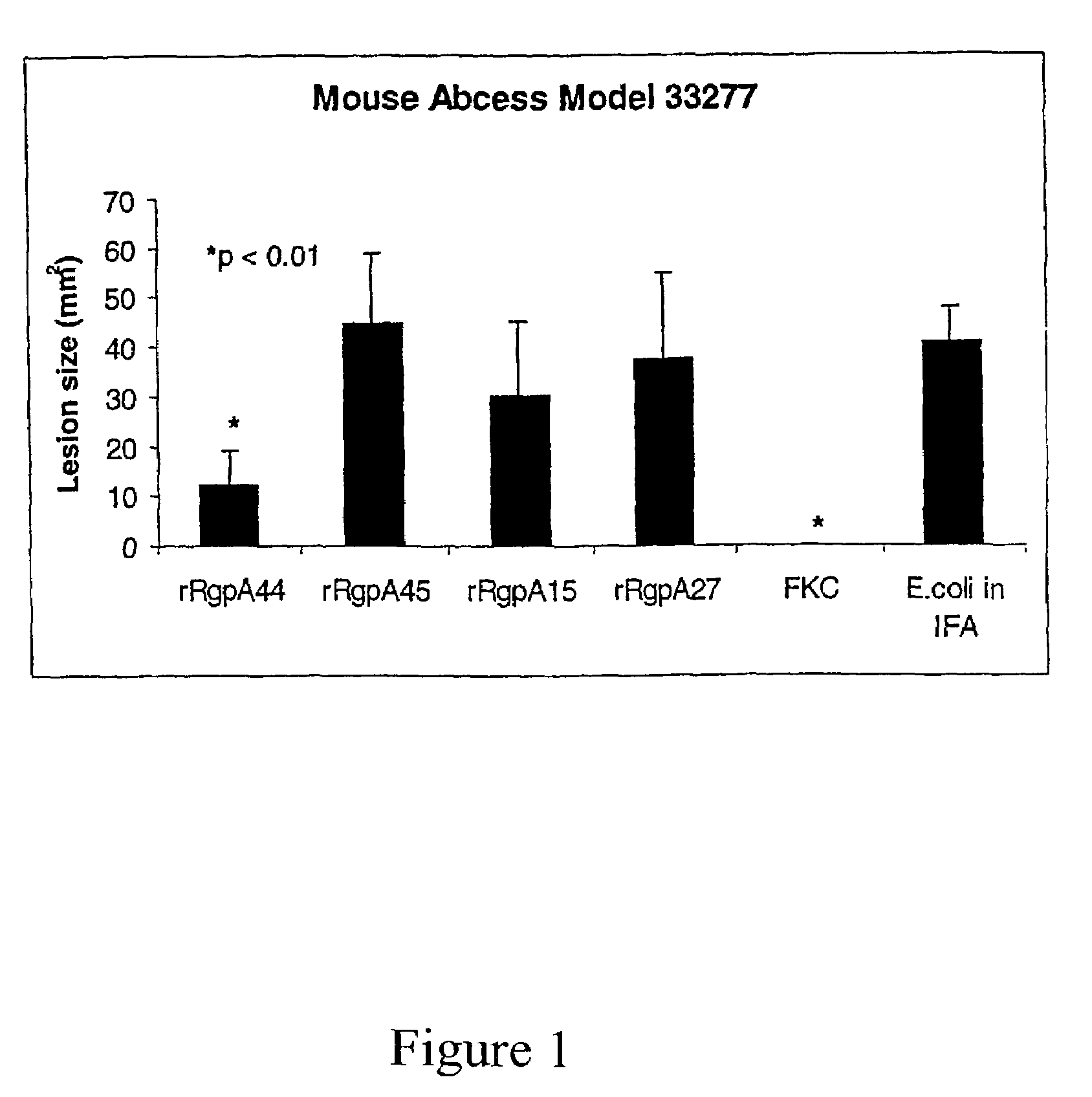
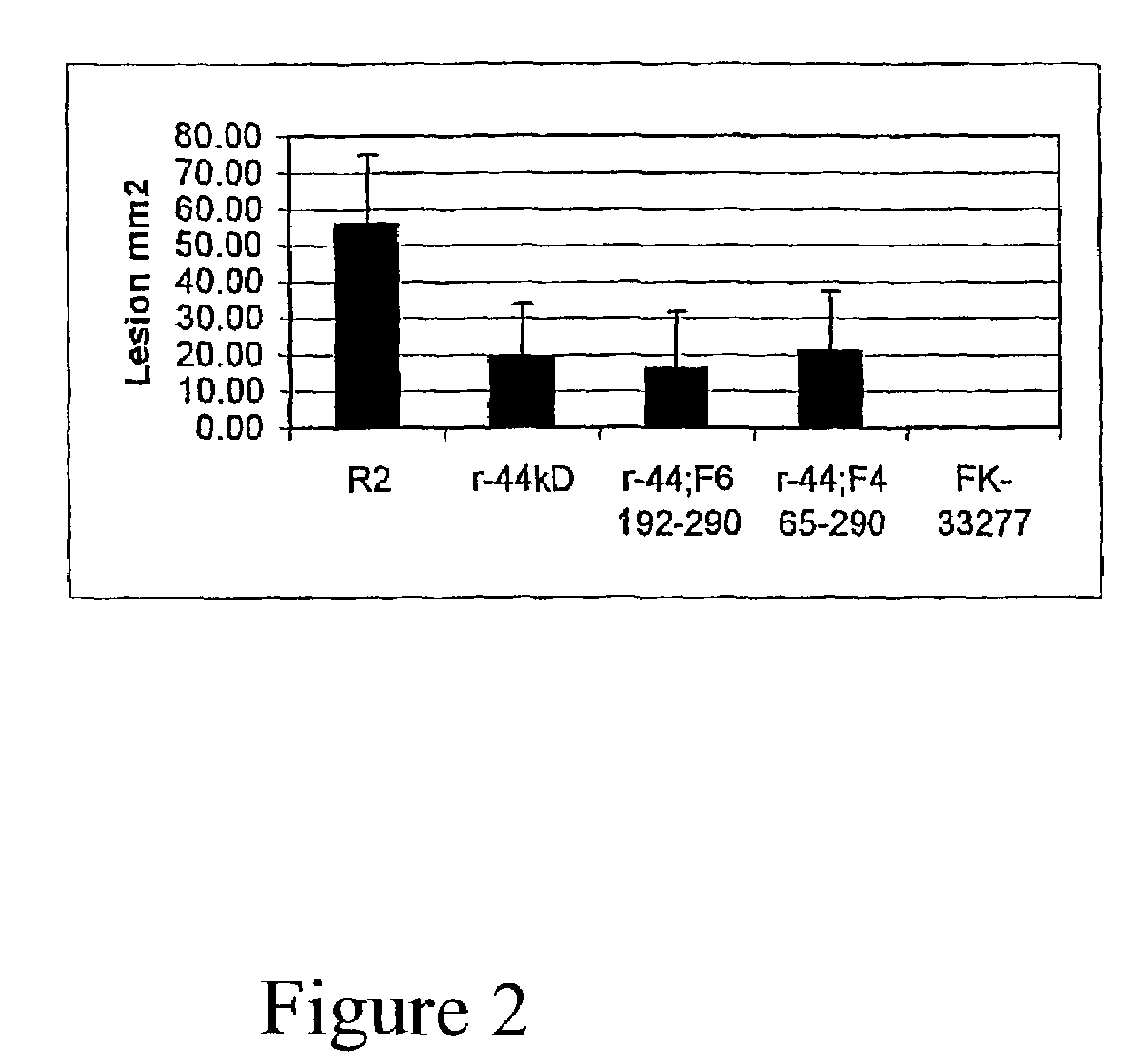
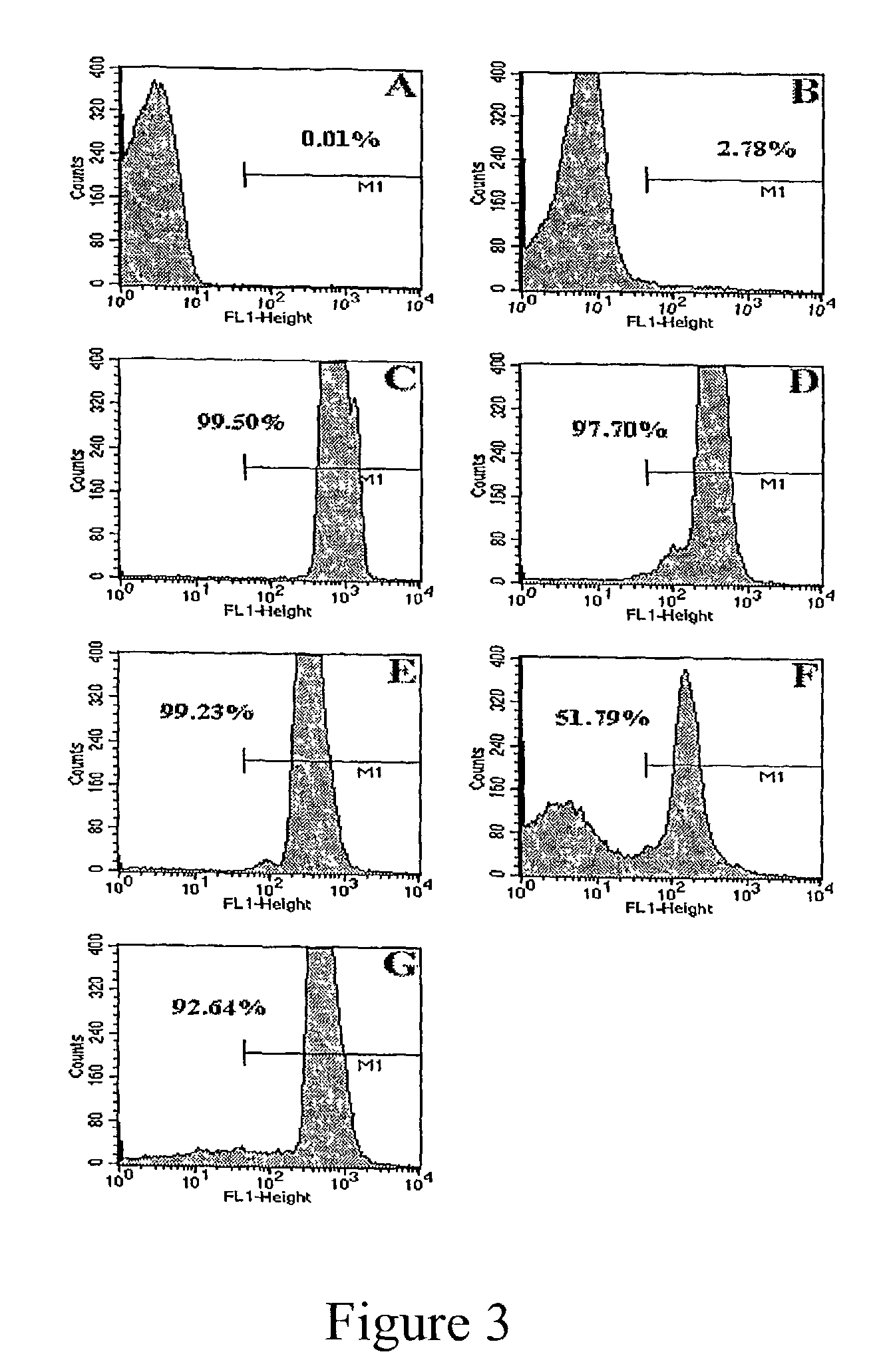
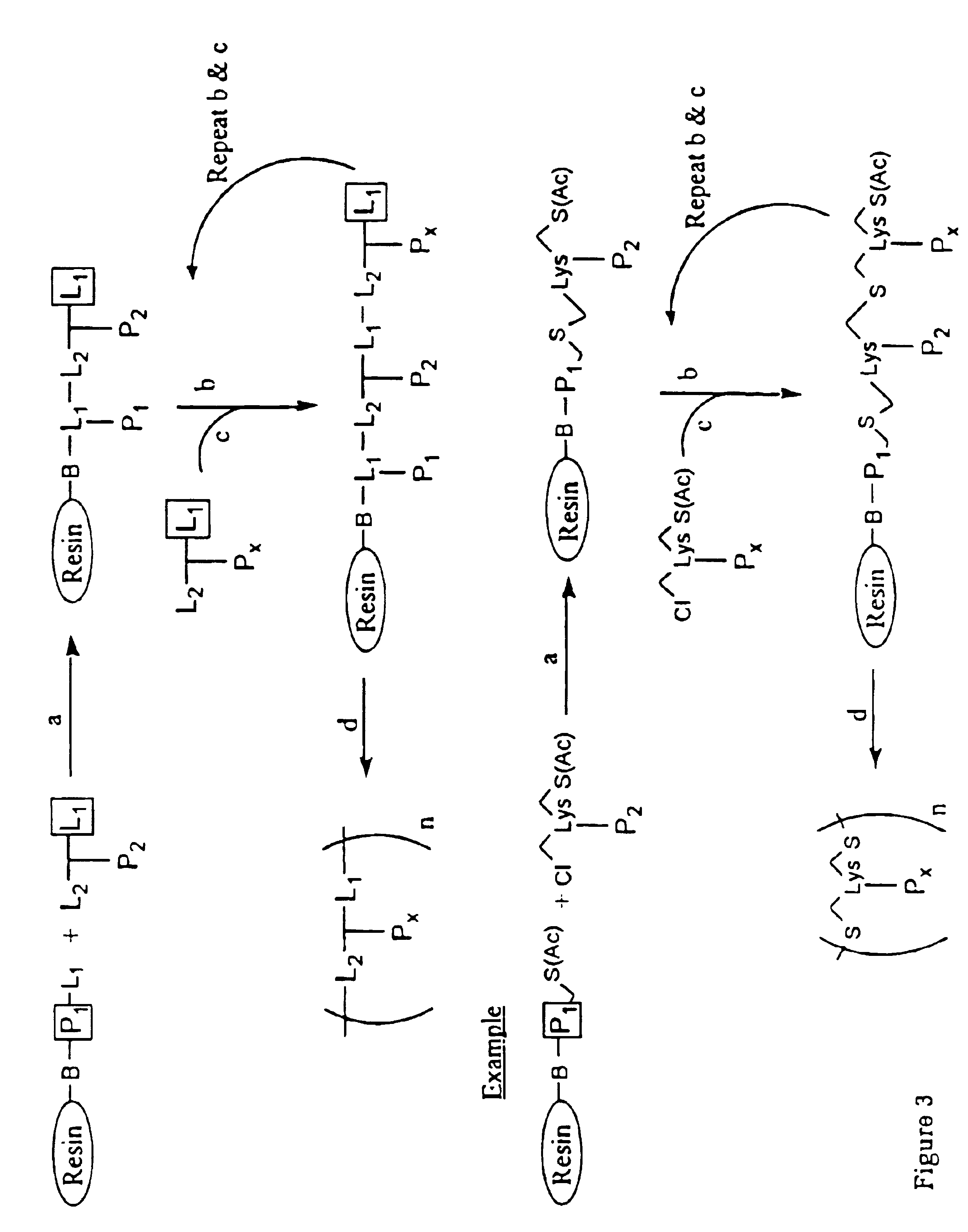
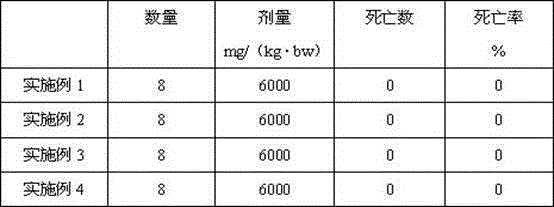
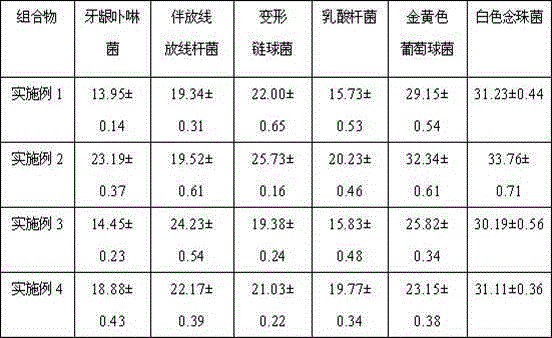
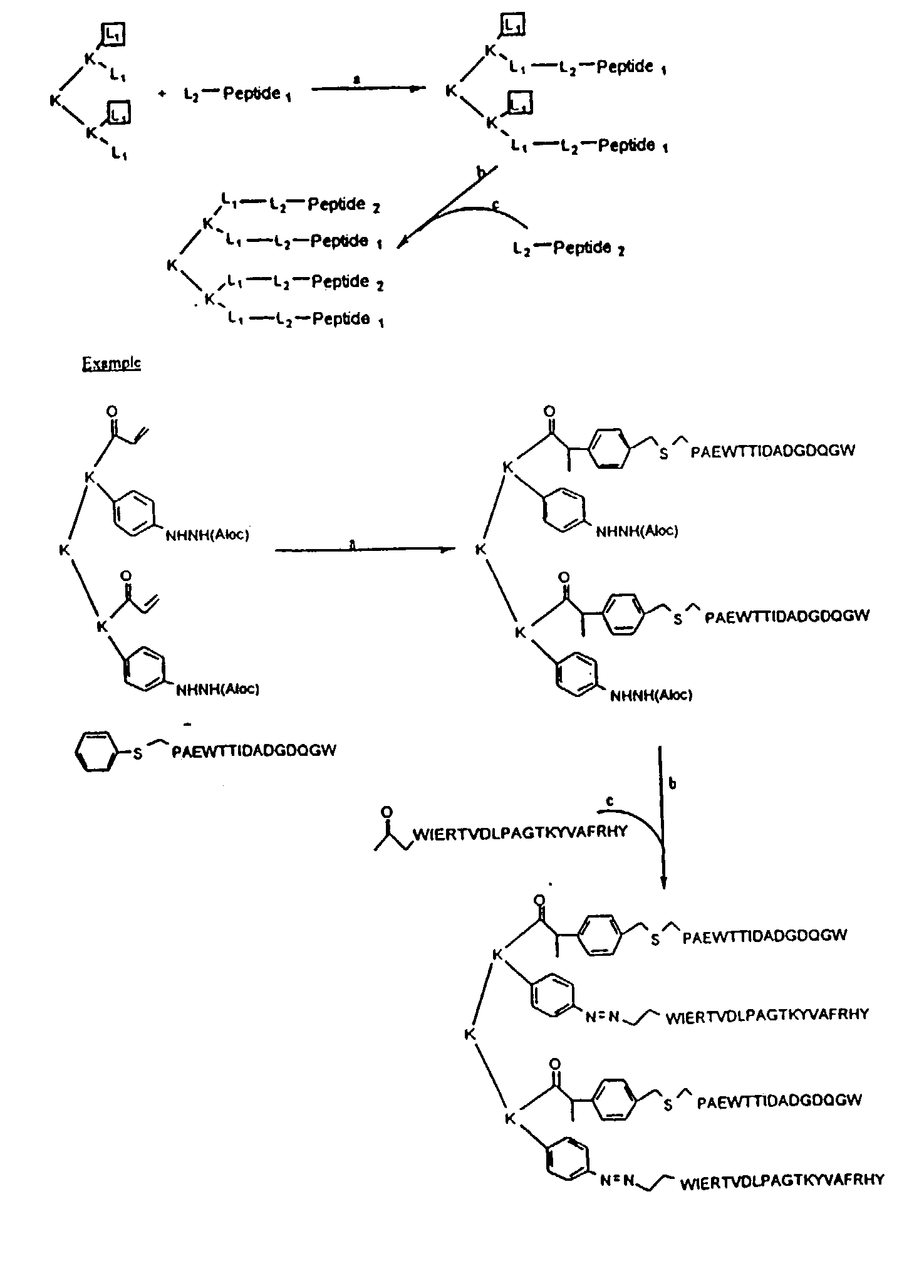
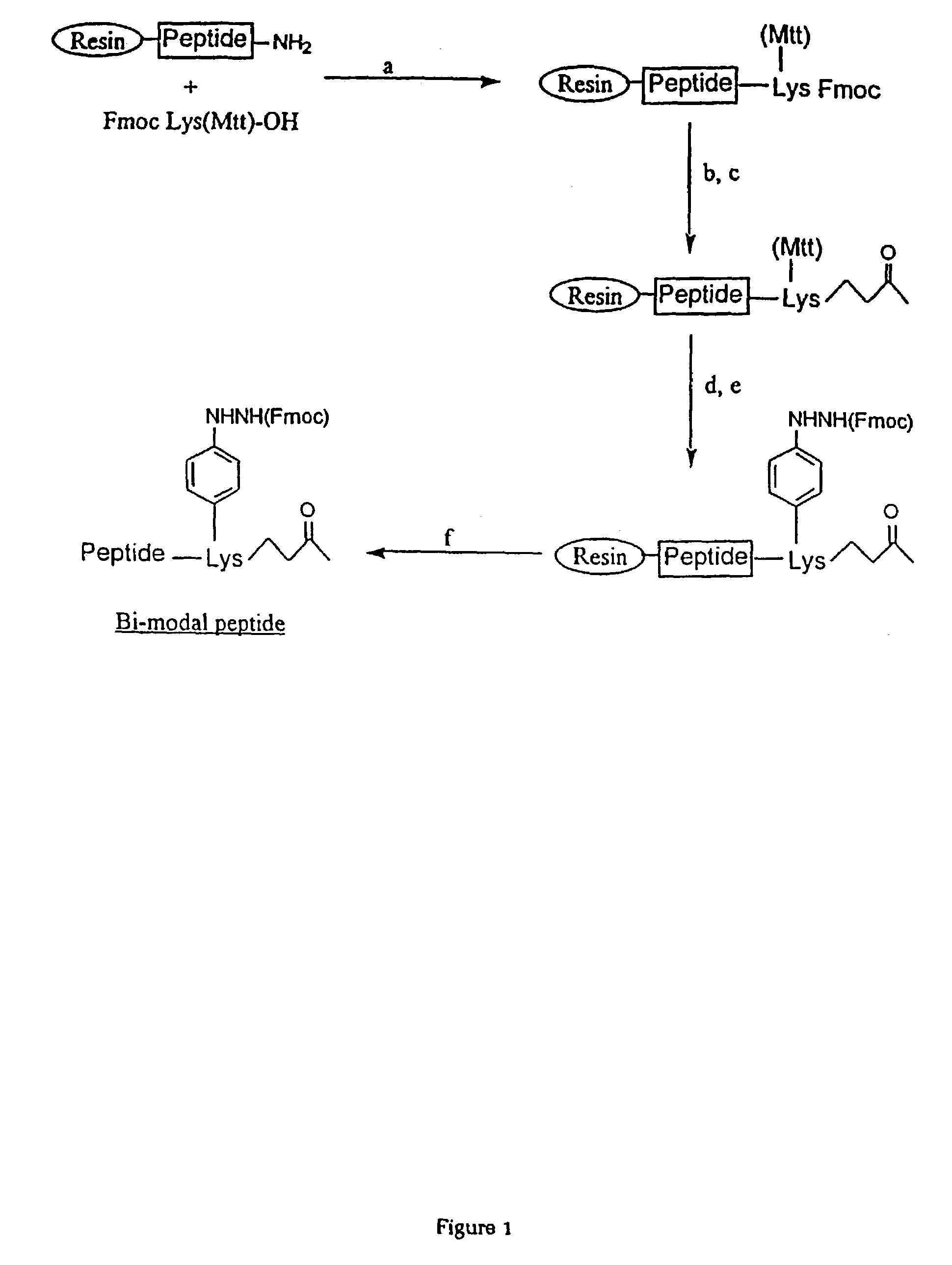
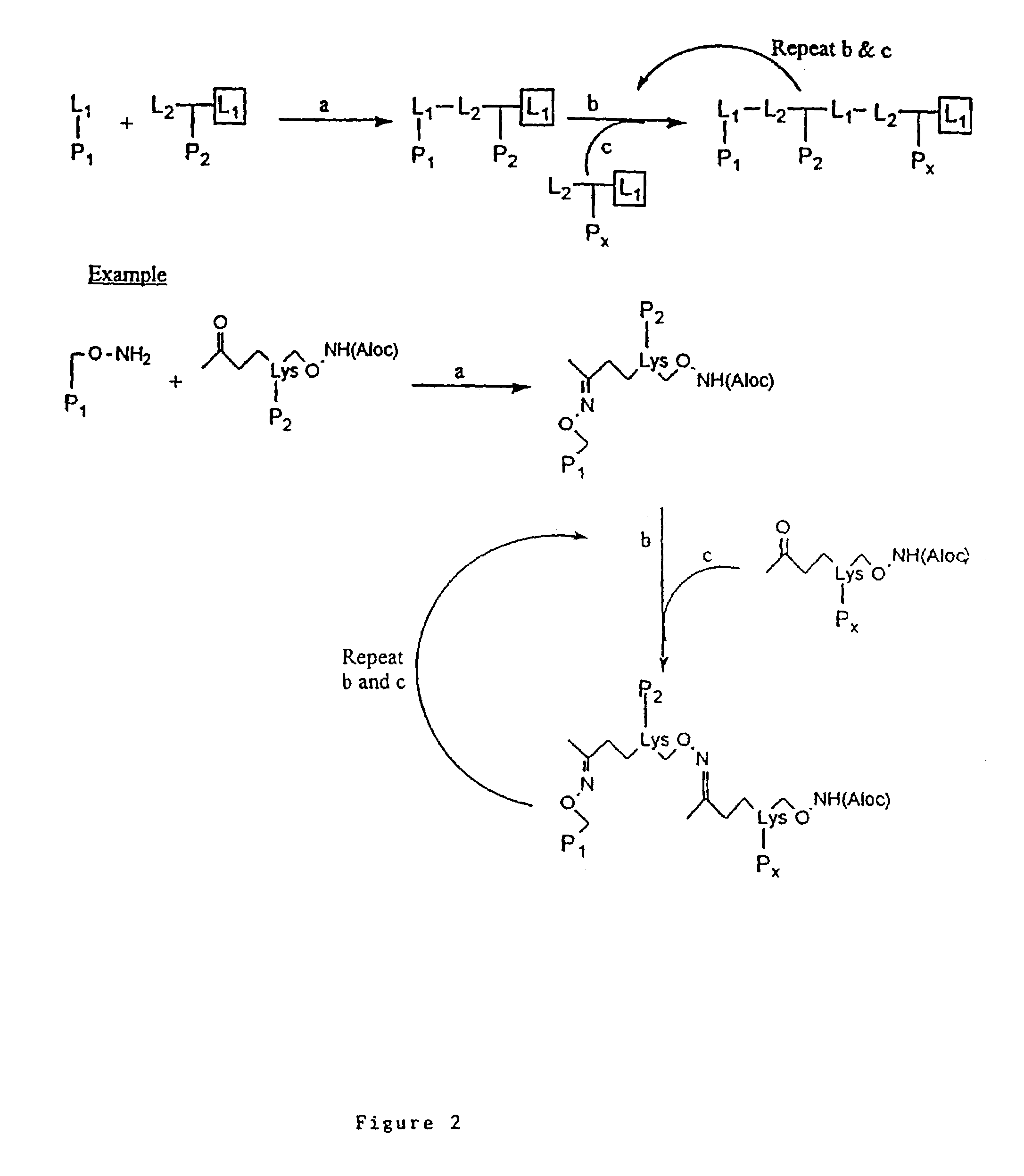
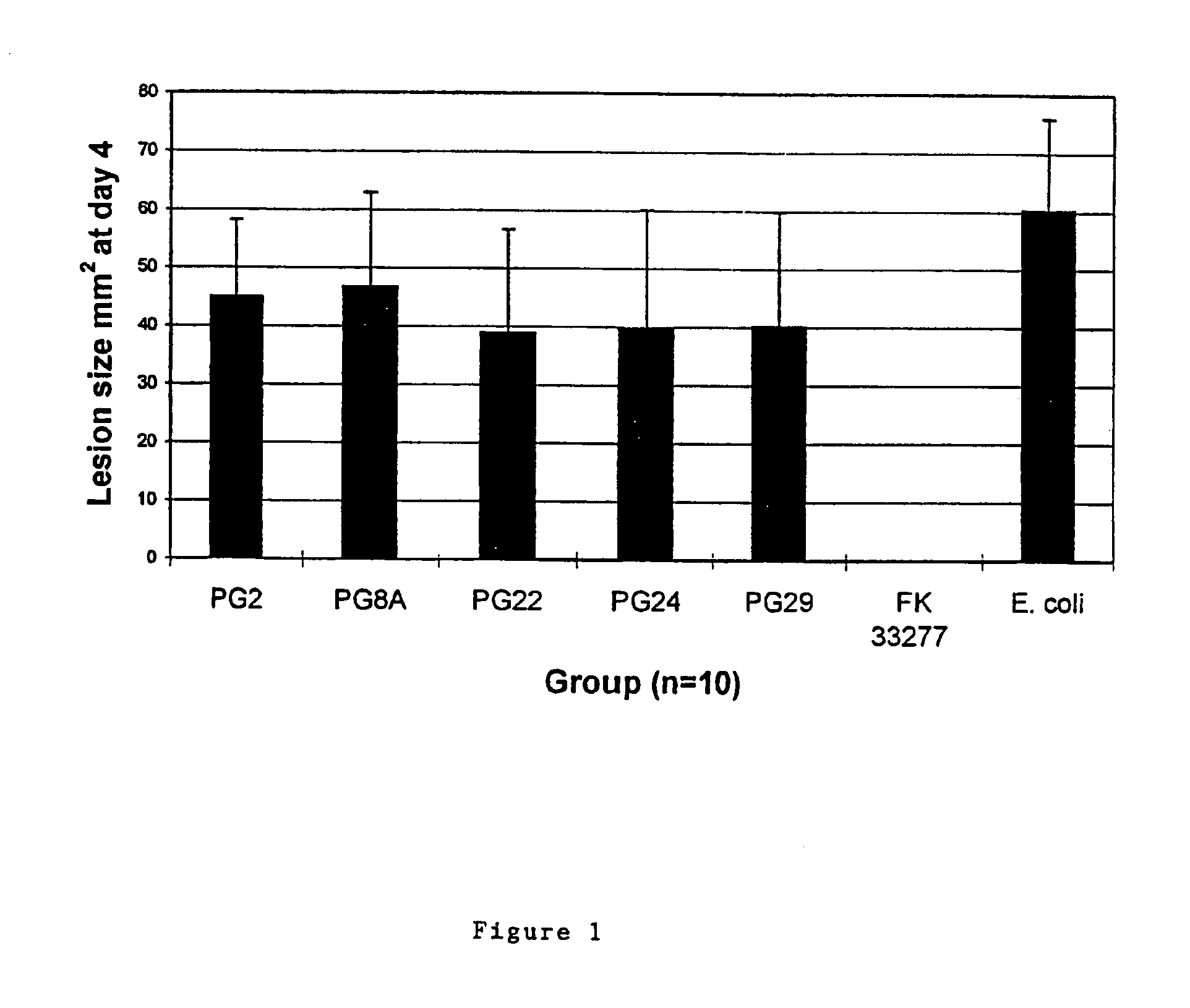
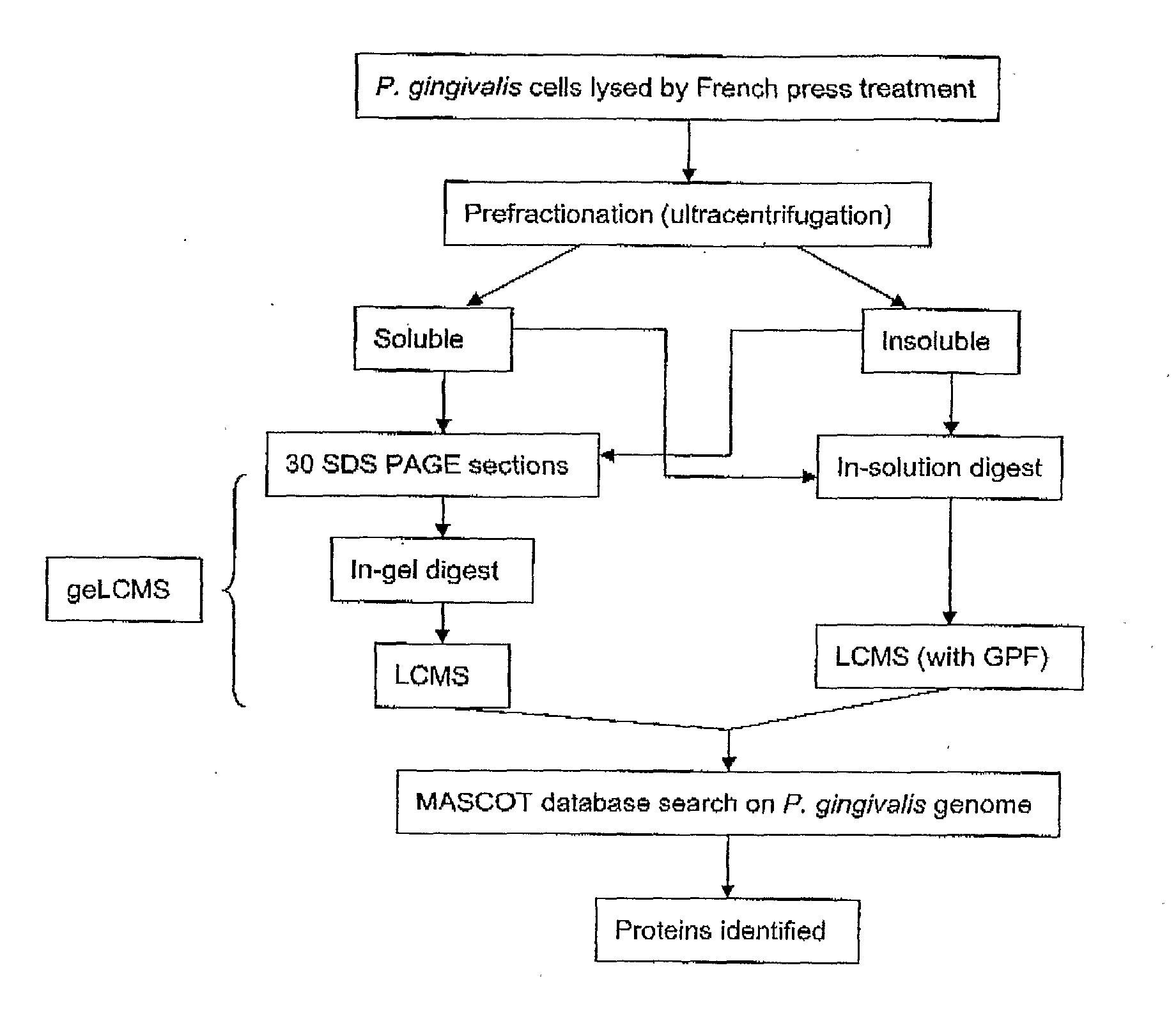
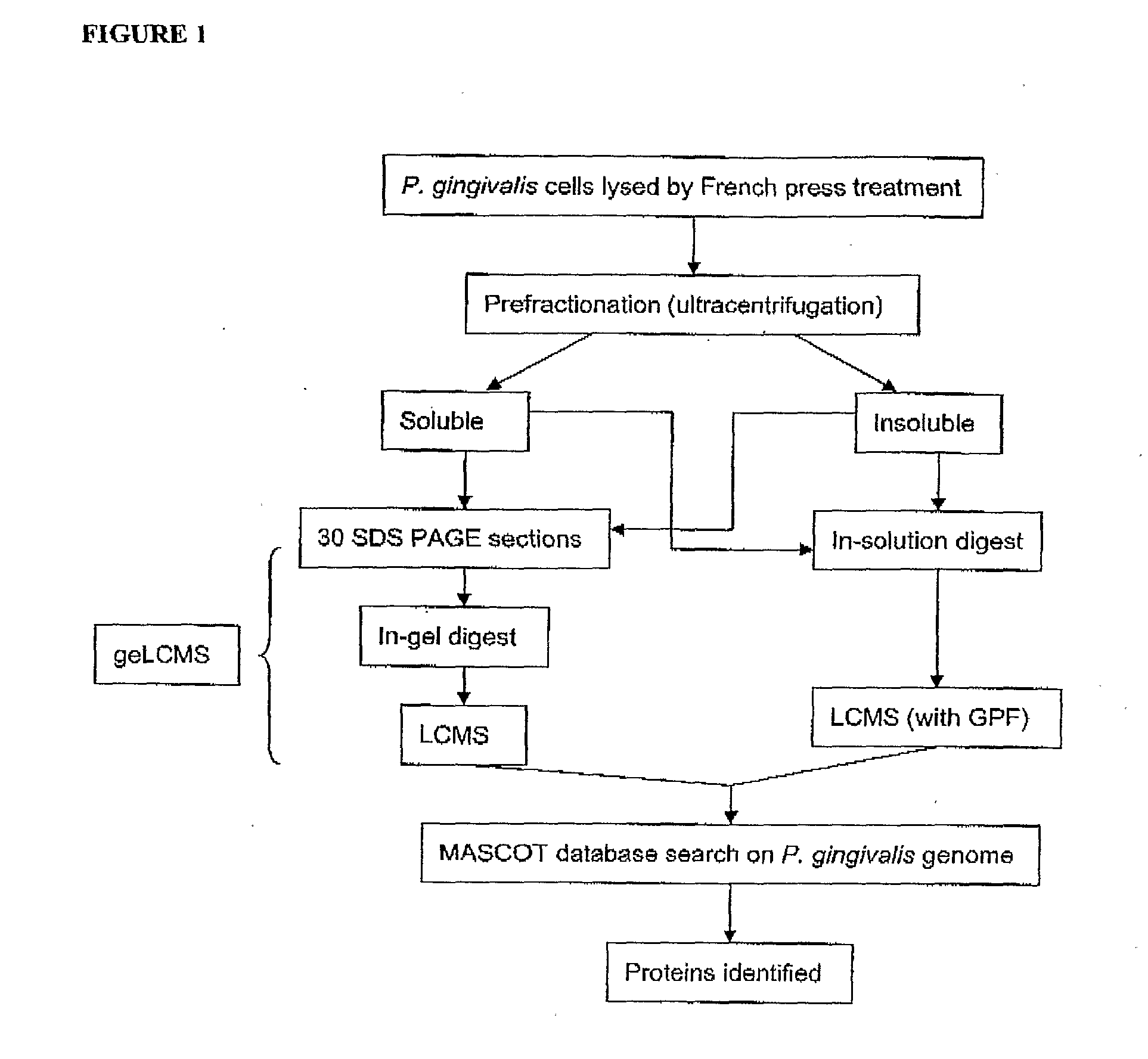
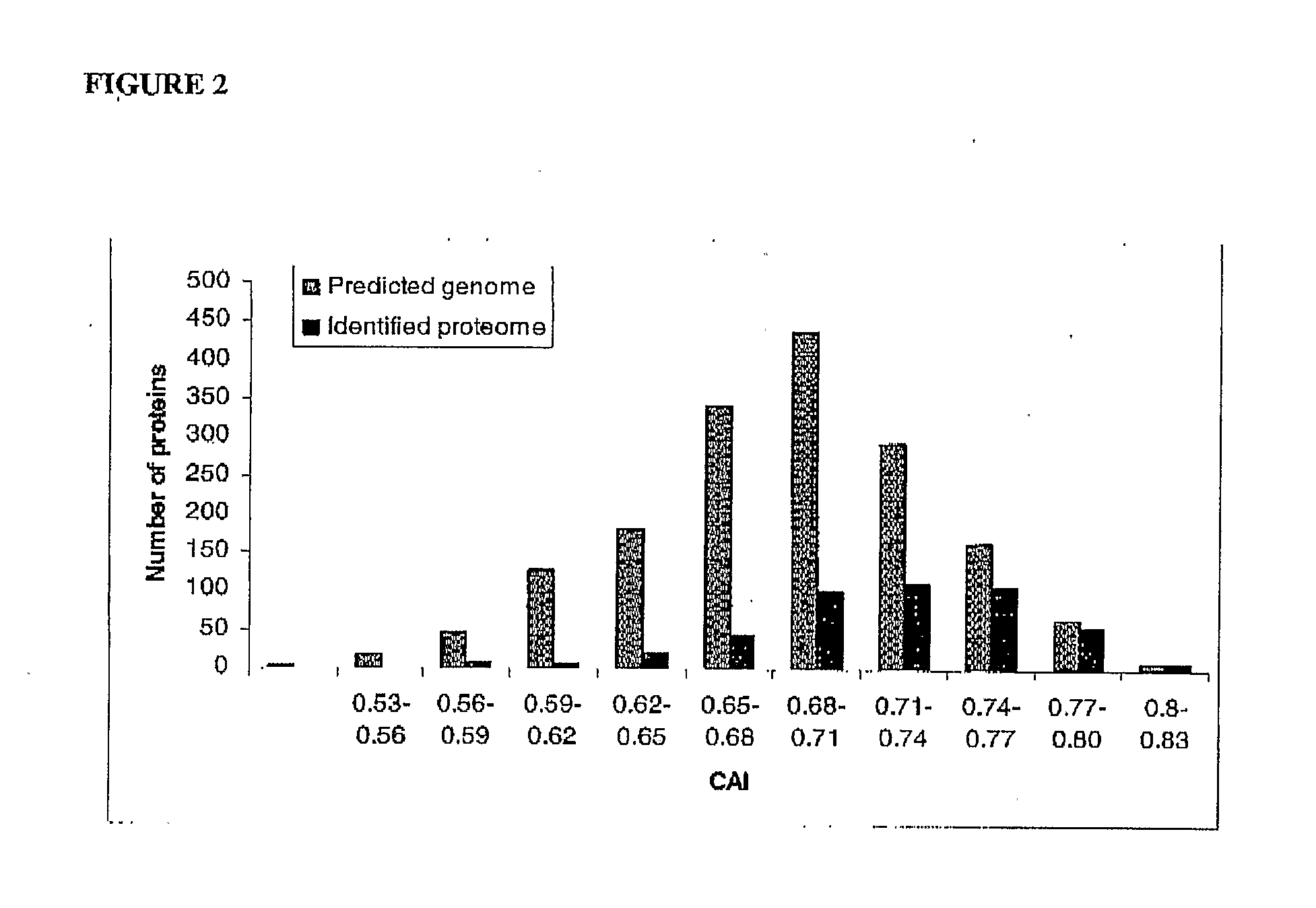
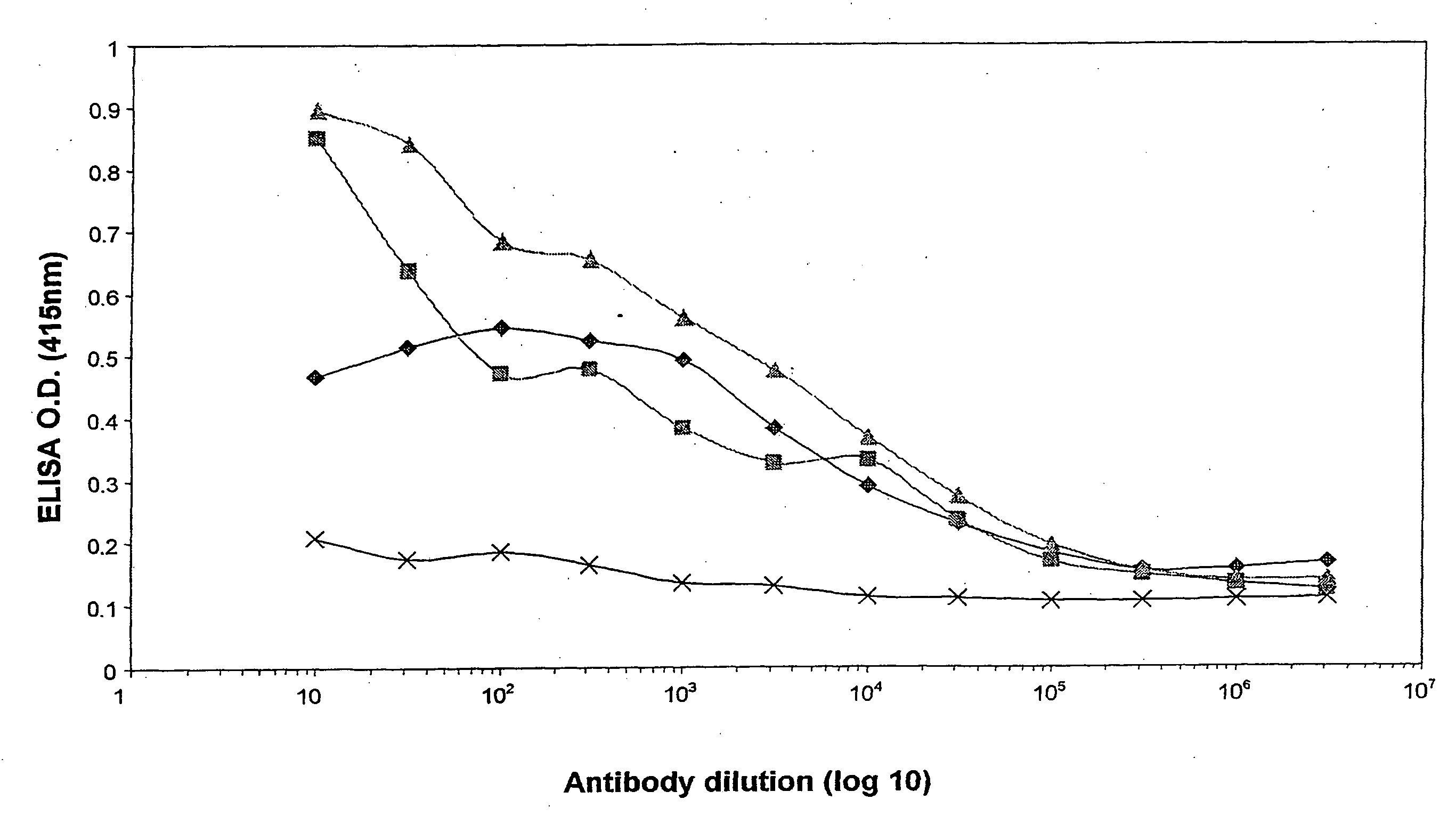
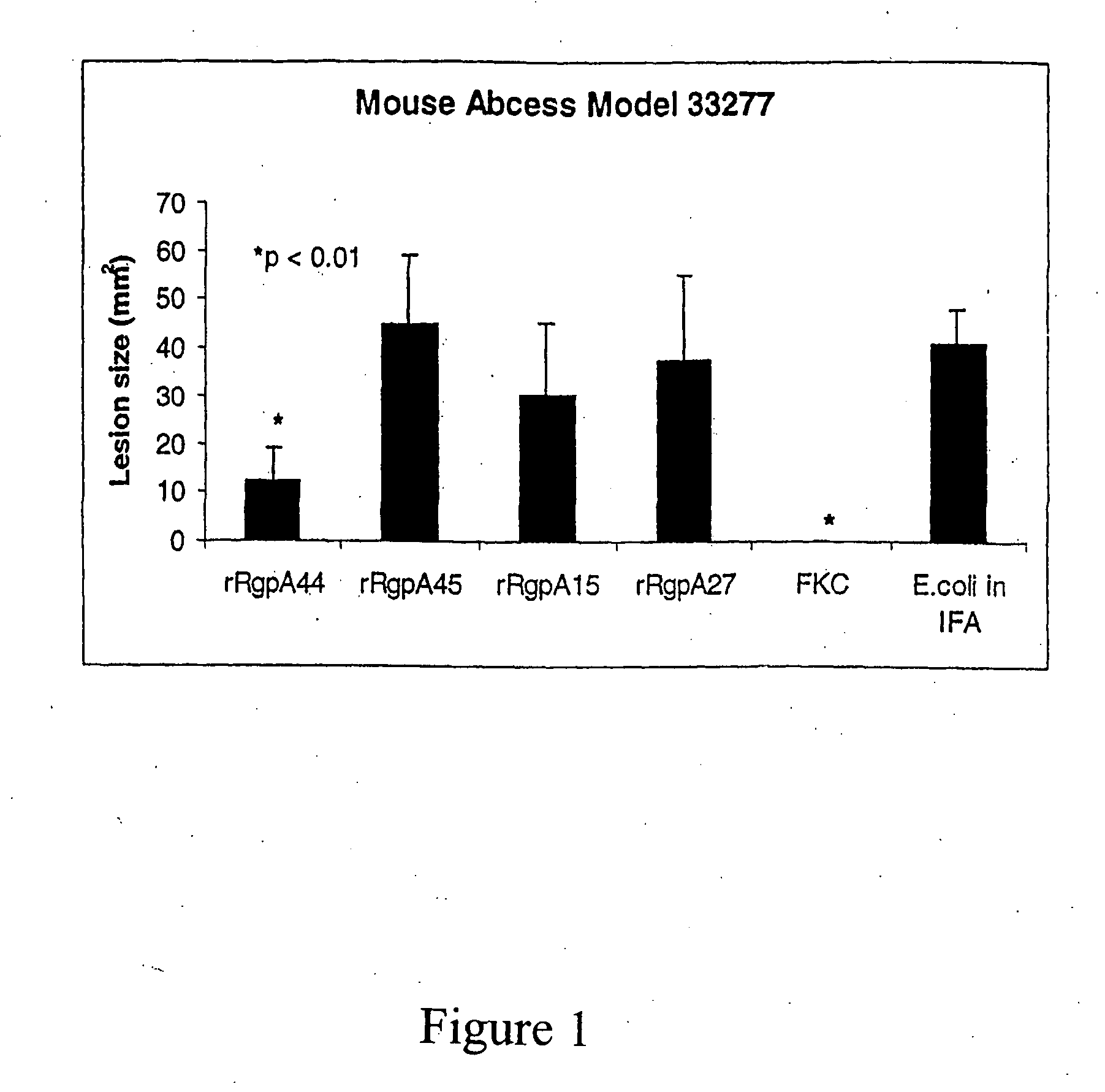
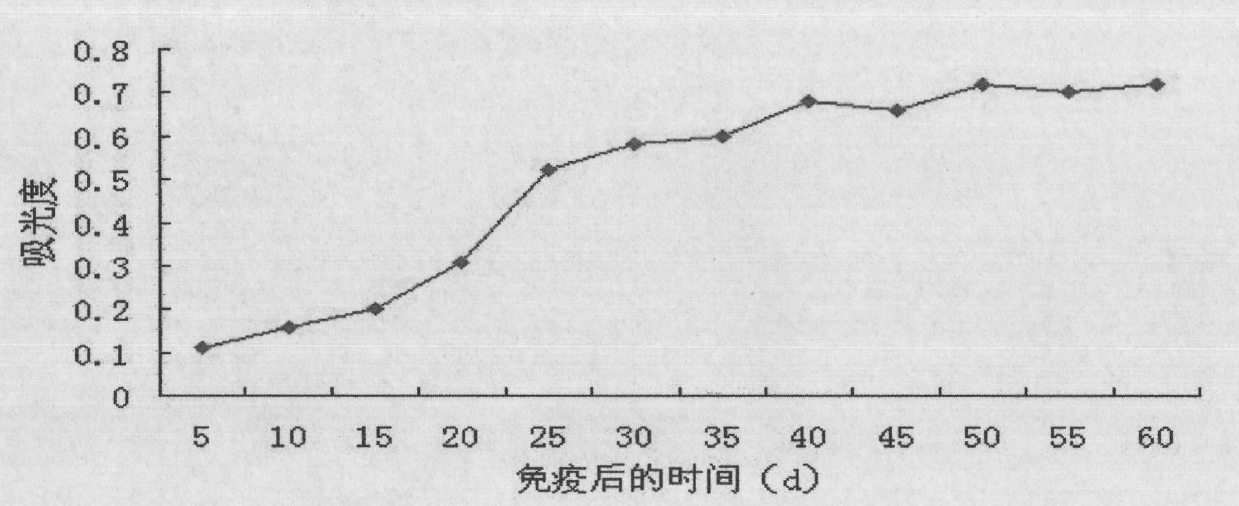
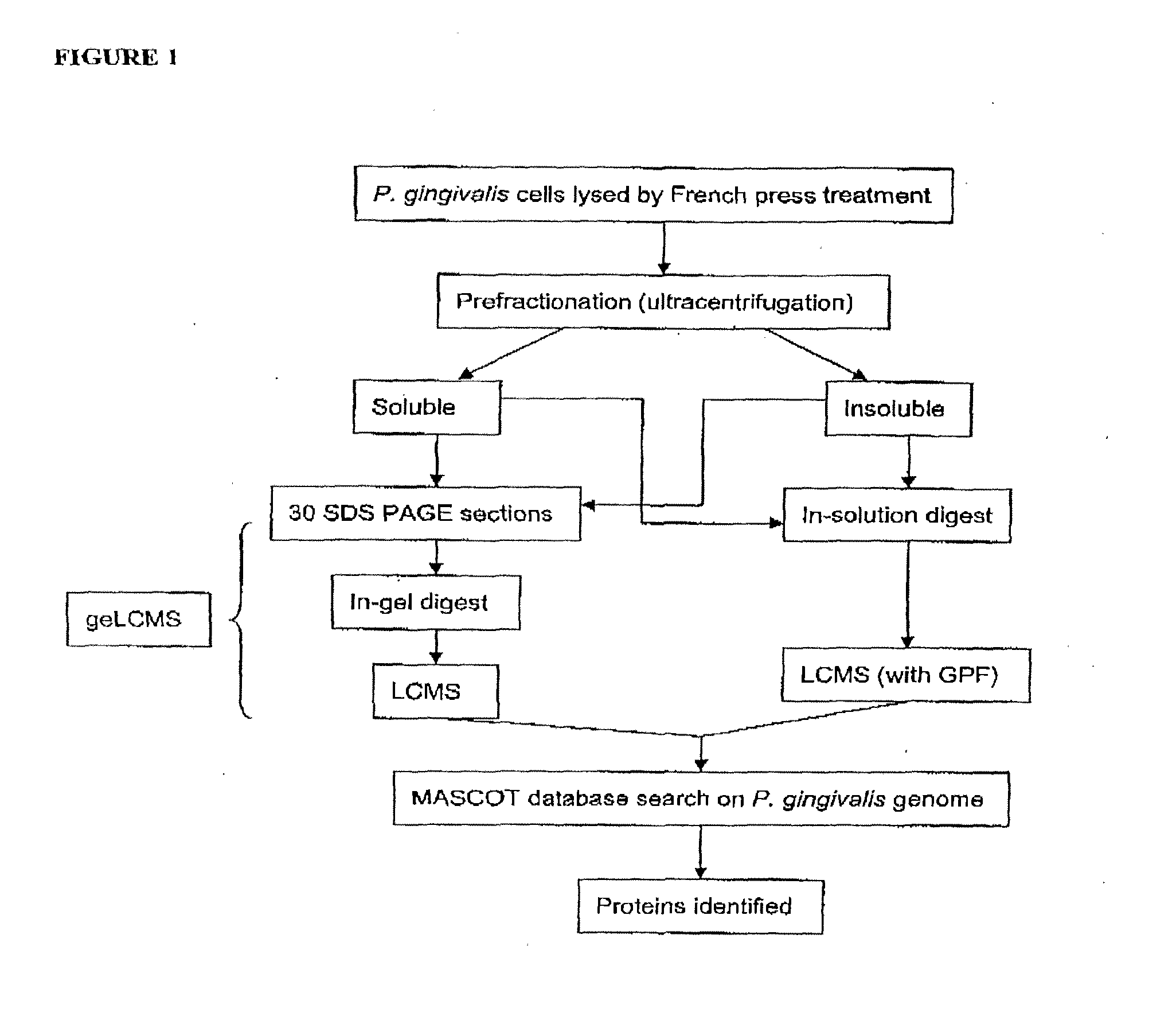
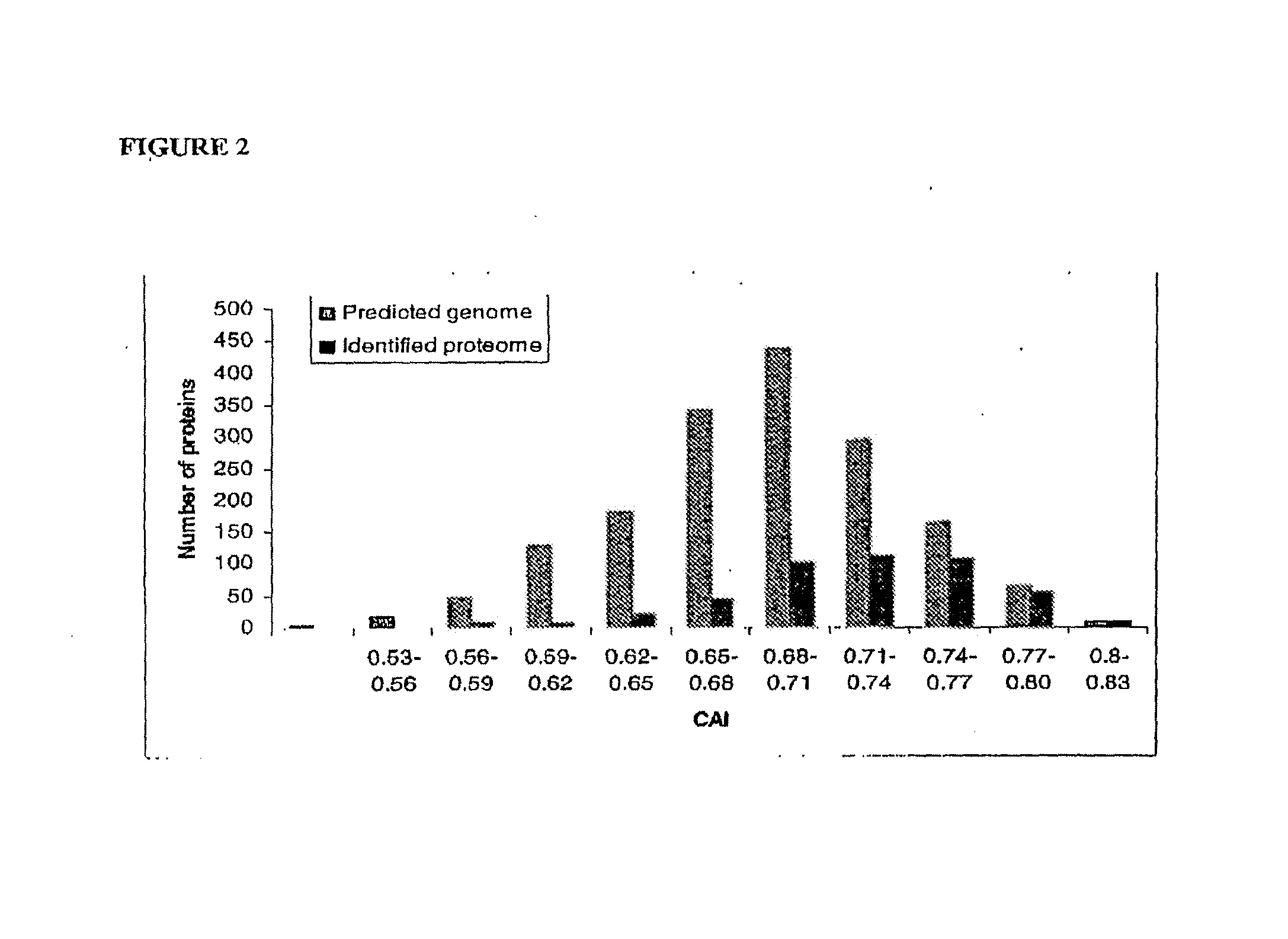
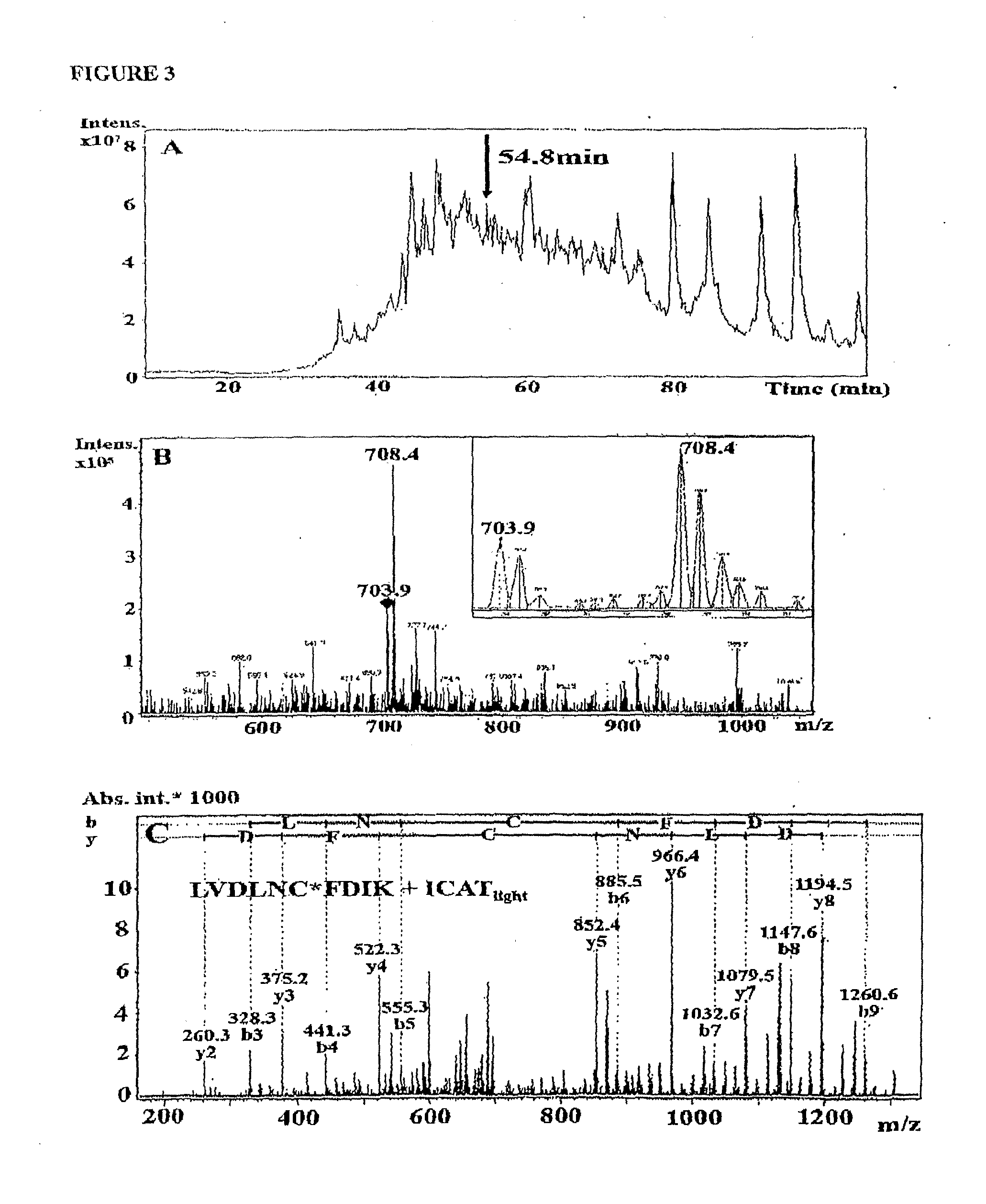
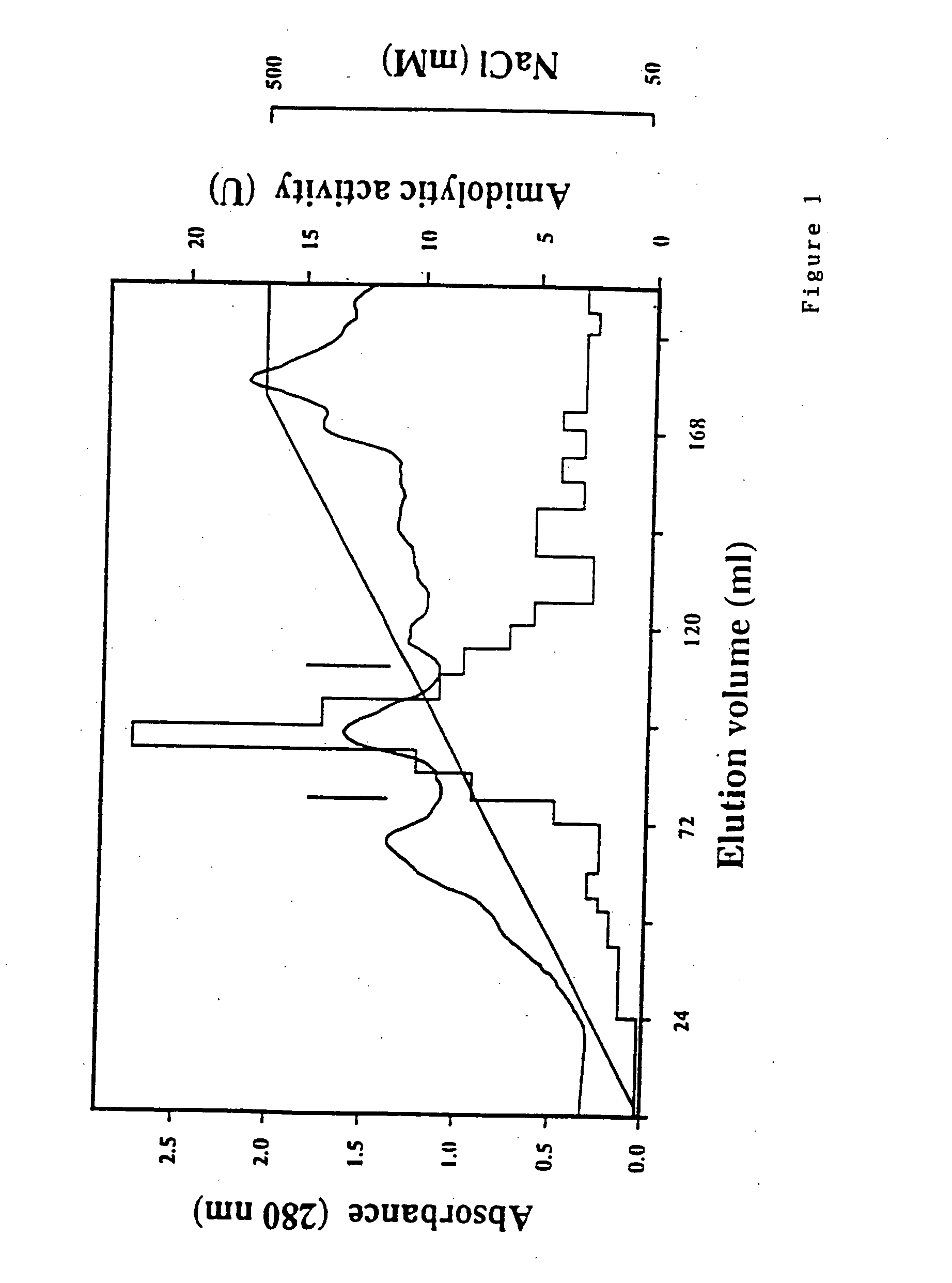
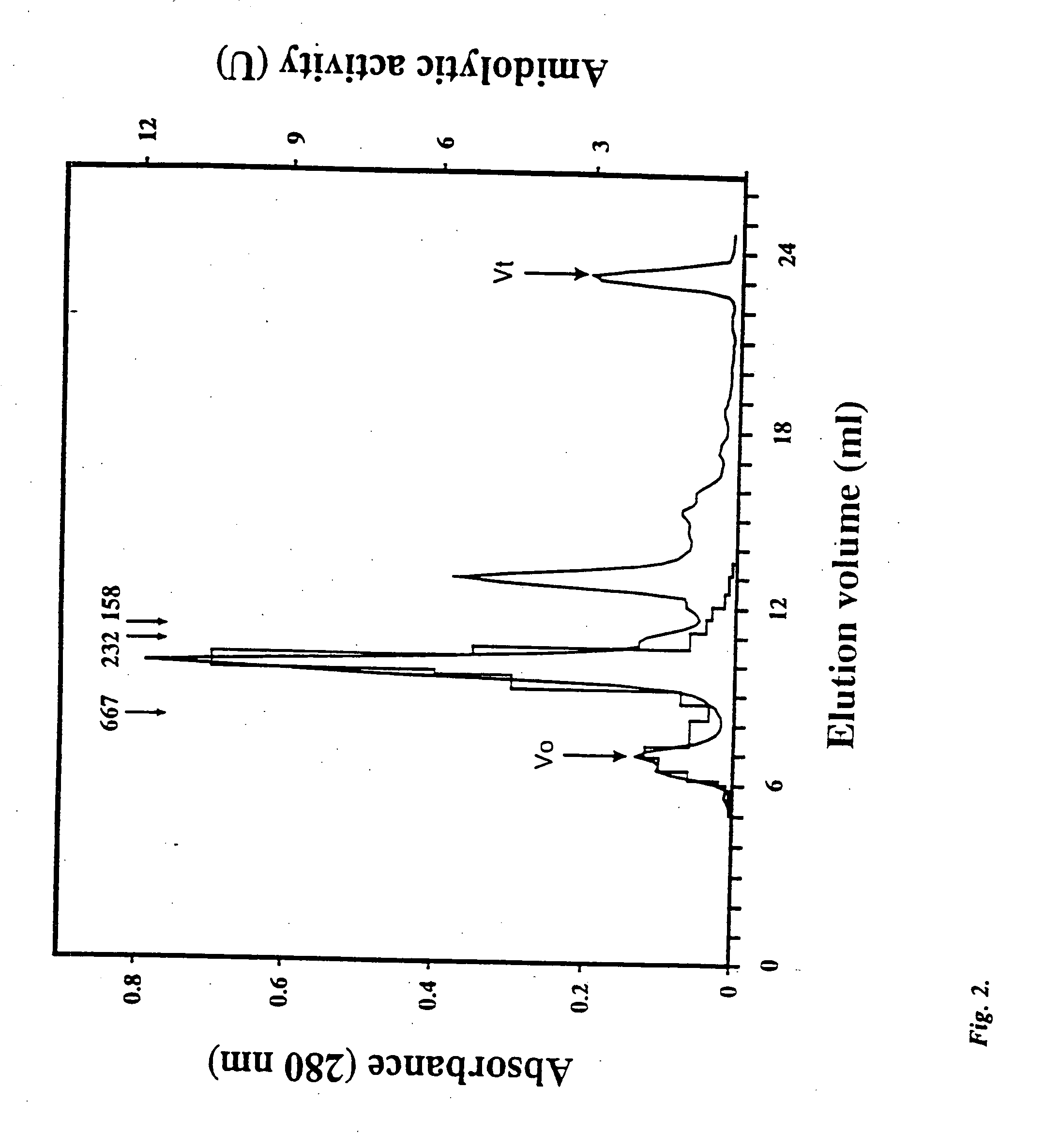
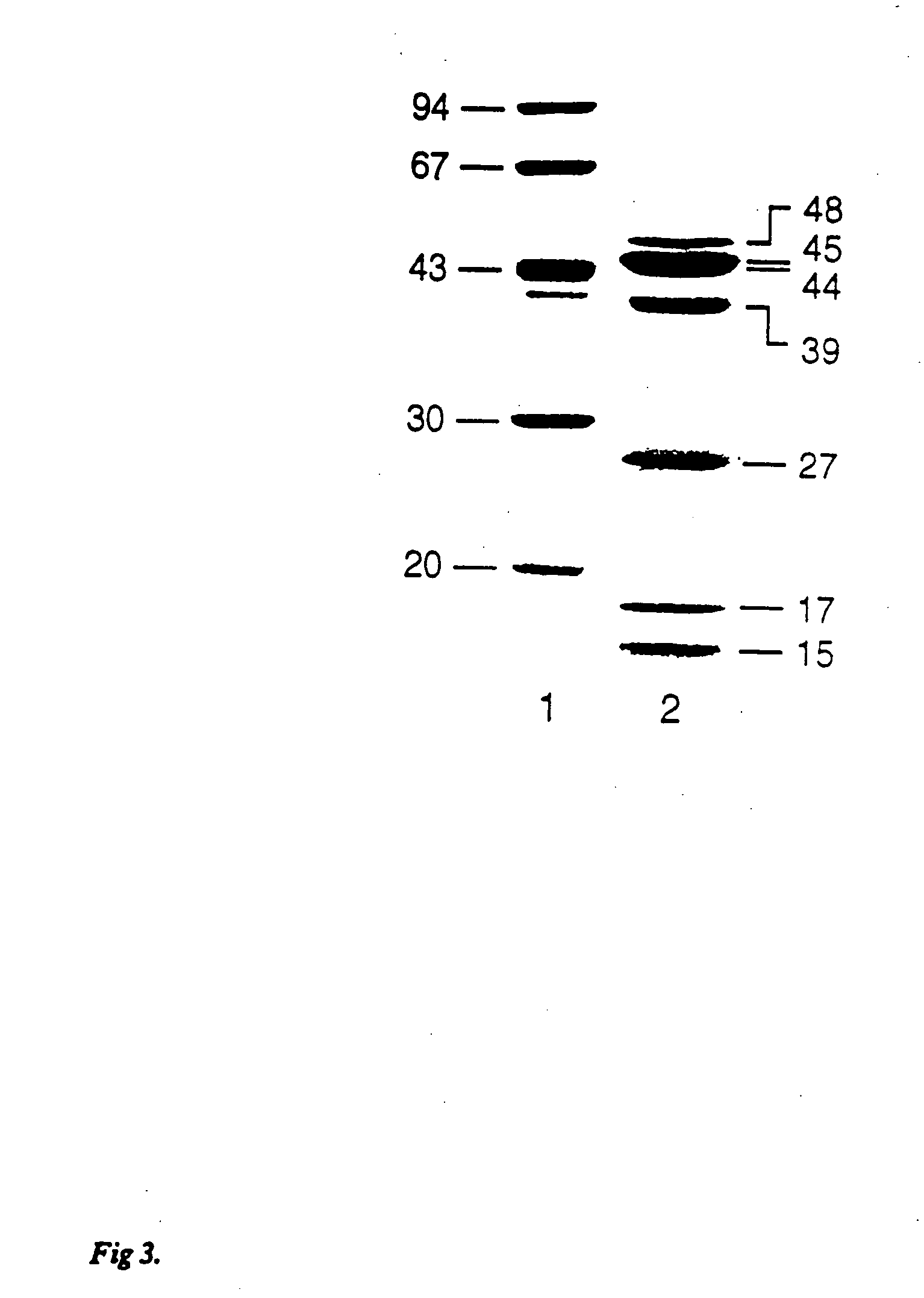
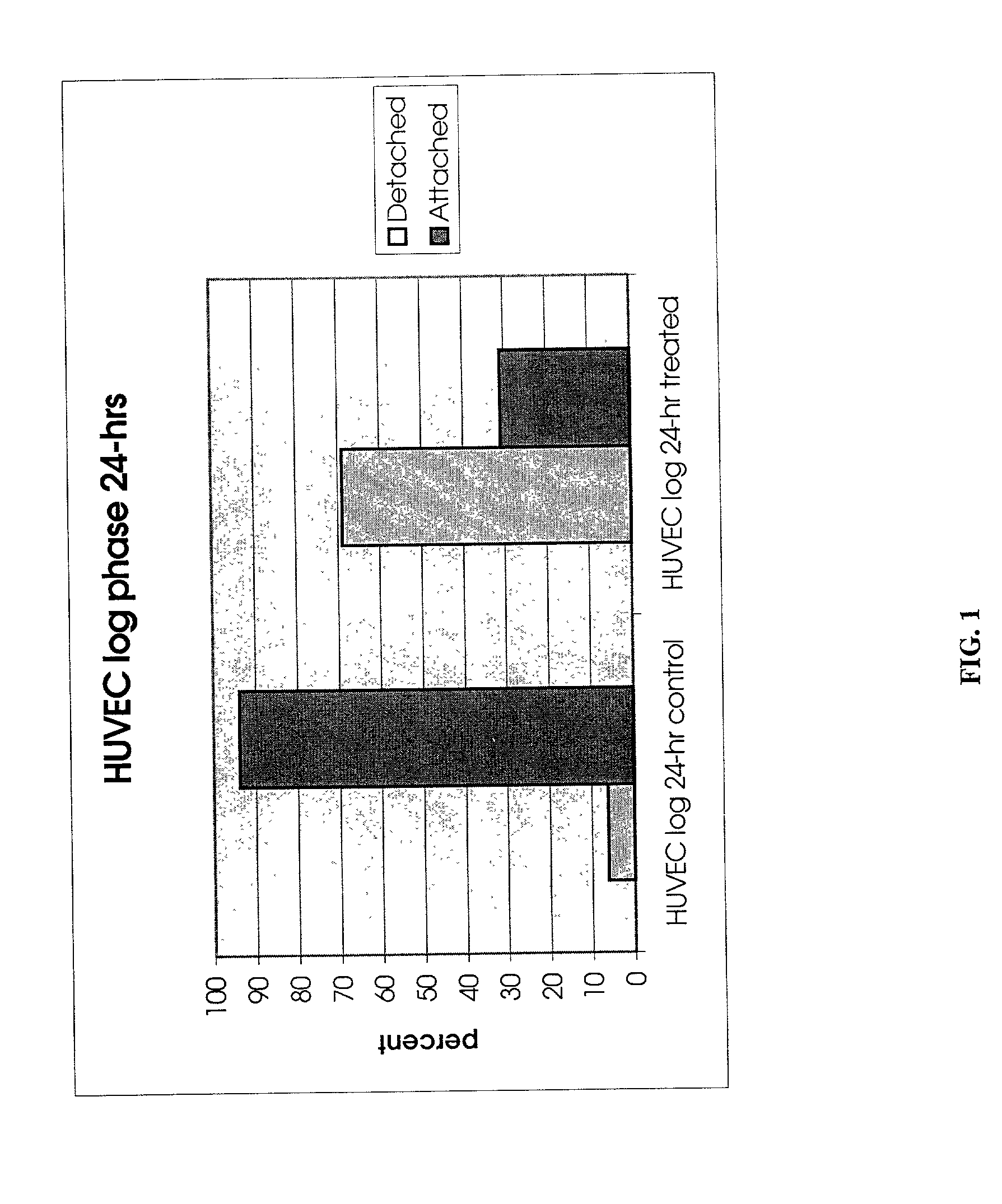
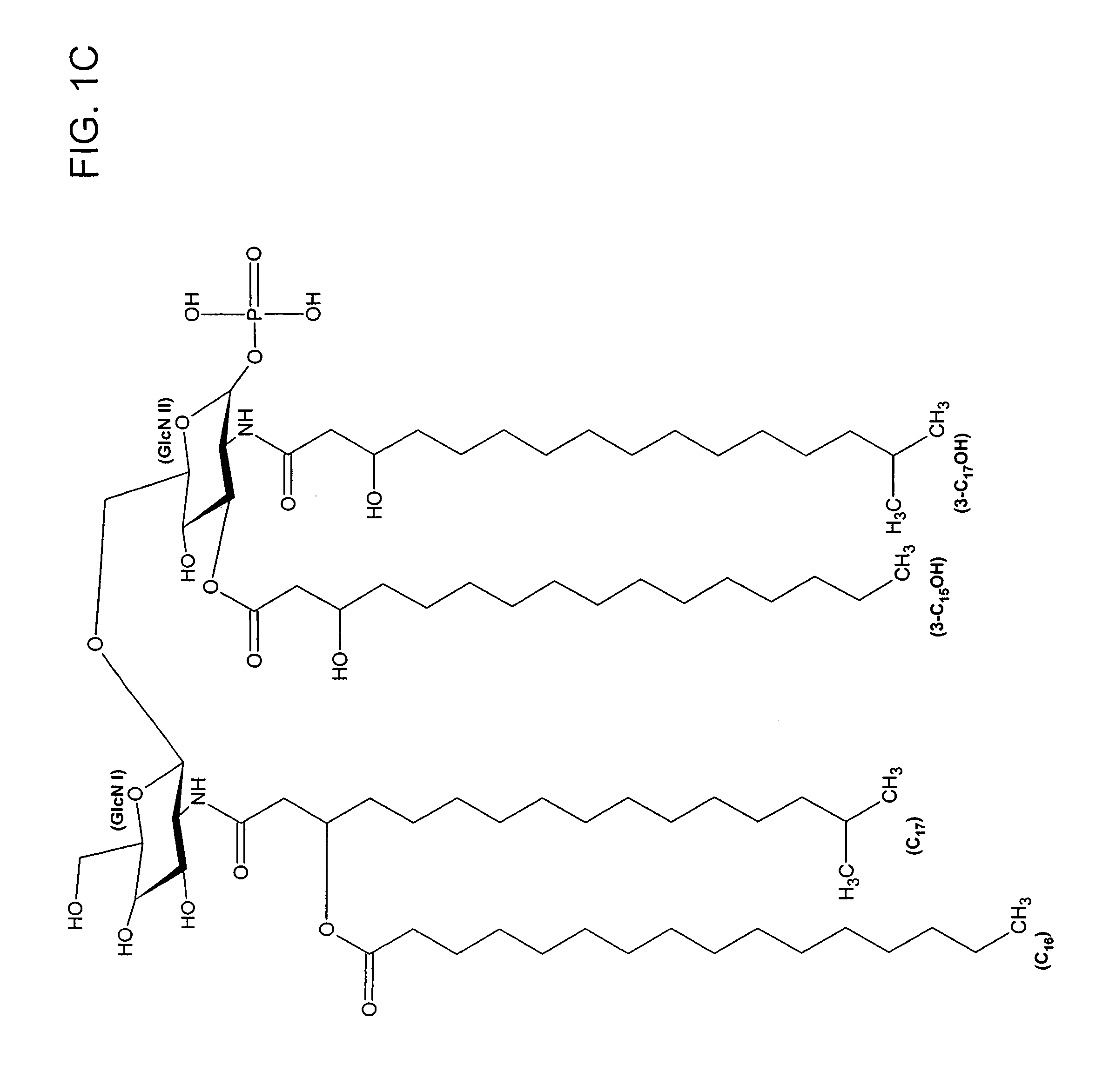
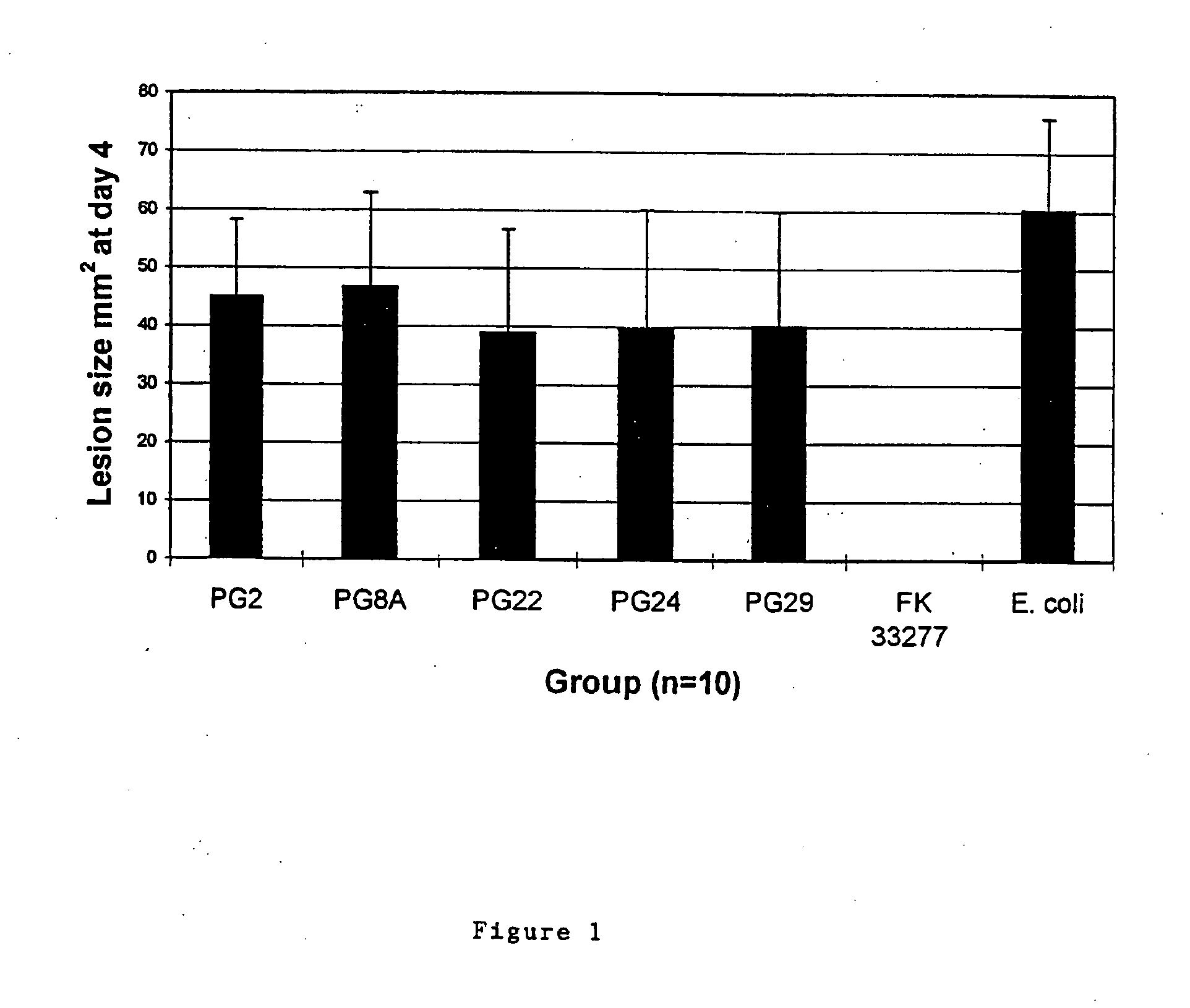
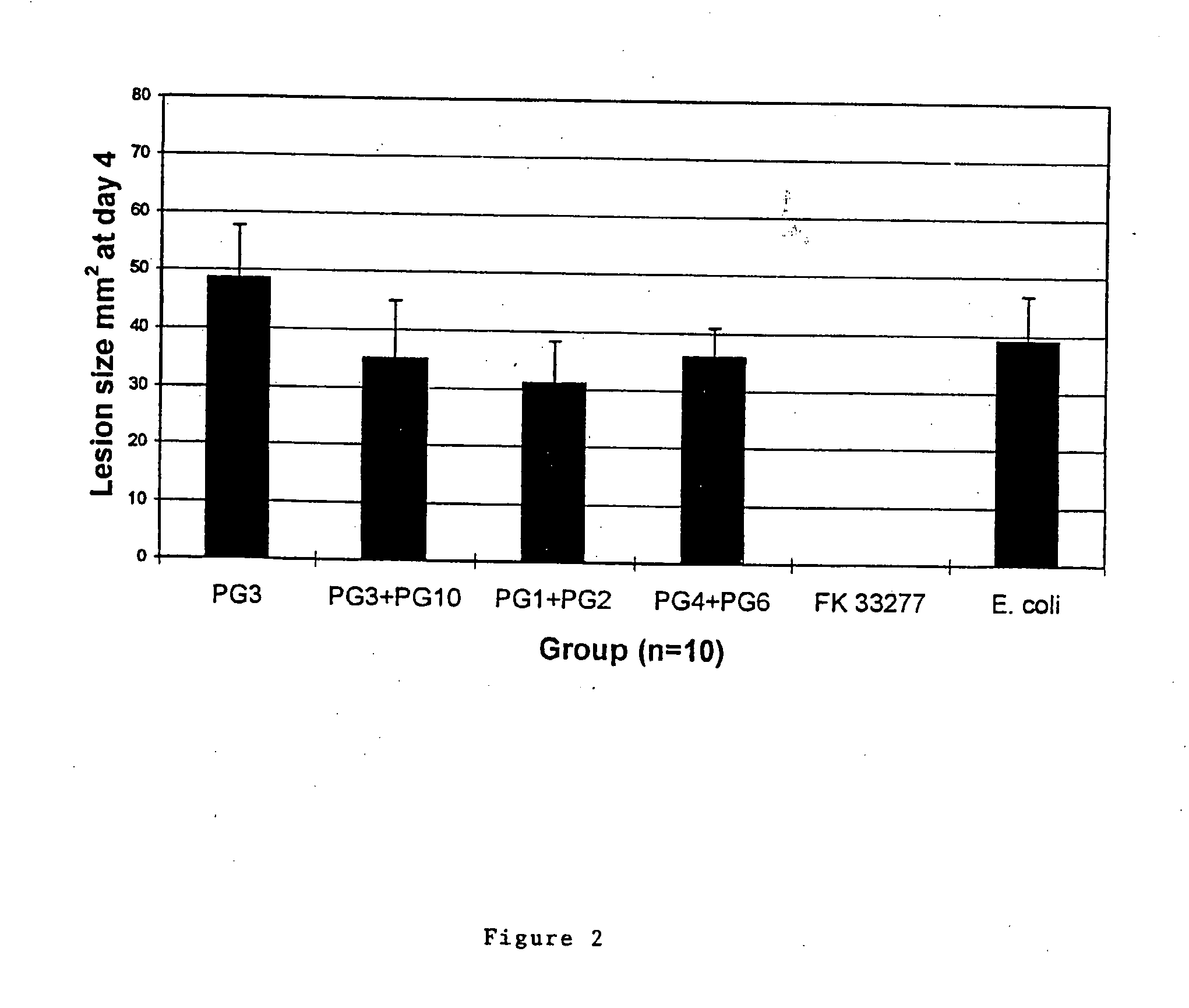
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
92 results about "Porphyromonas gingivalis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Porphyromonas gingivalis belongs to the phylum Bacteroidetes and is a nonmotile, Gram-negative, rod-shaped, anaerobic, pathogenic bacterium. It forms black colonies on blood agar. It is found in the oral cavity, where it is implicated in periodontal disease, as well as in the upper gastrointestinal tract, the respiratory tract and the colon. It has been isolated from women with bacterial vaginosis.
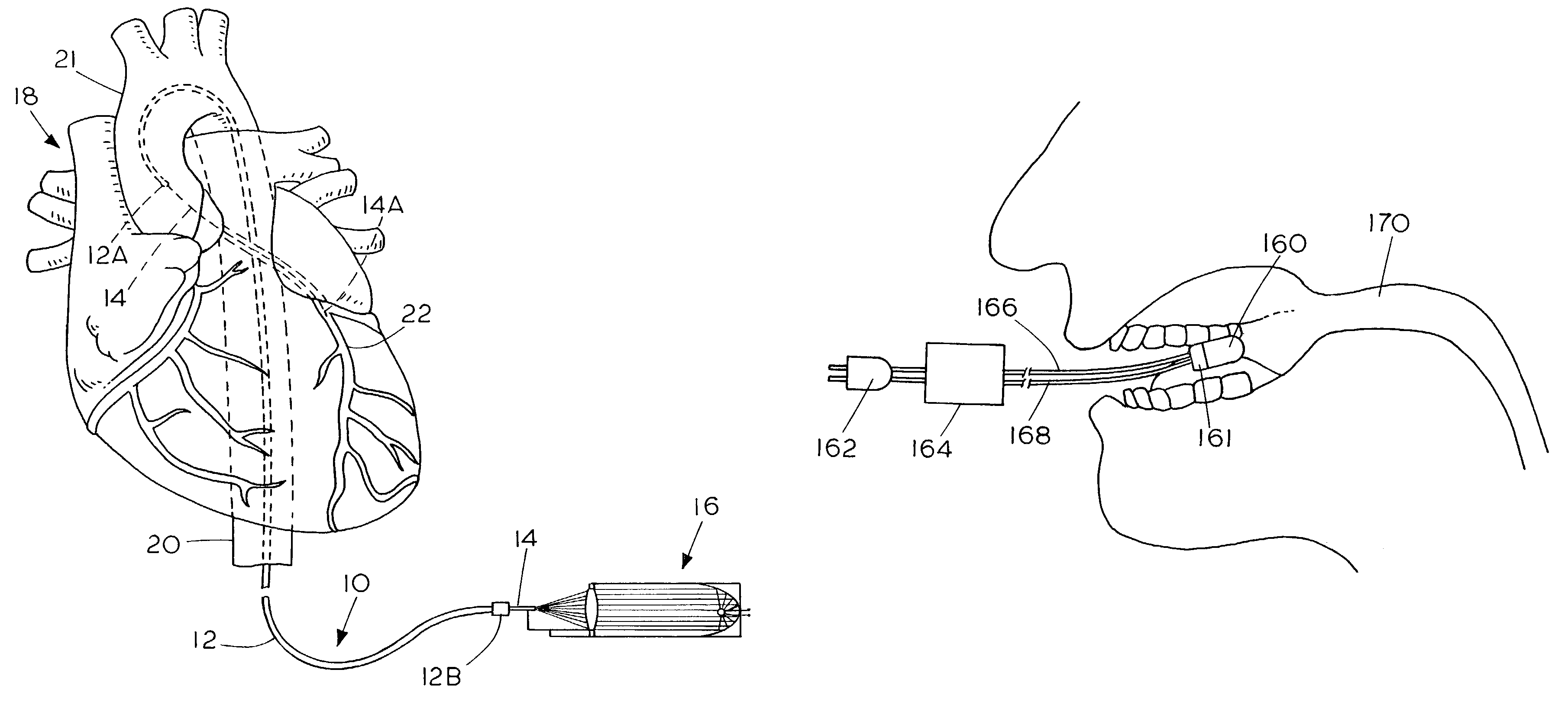
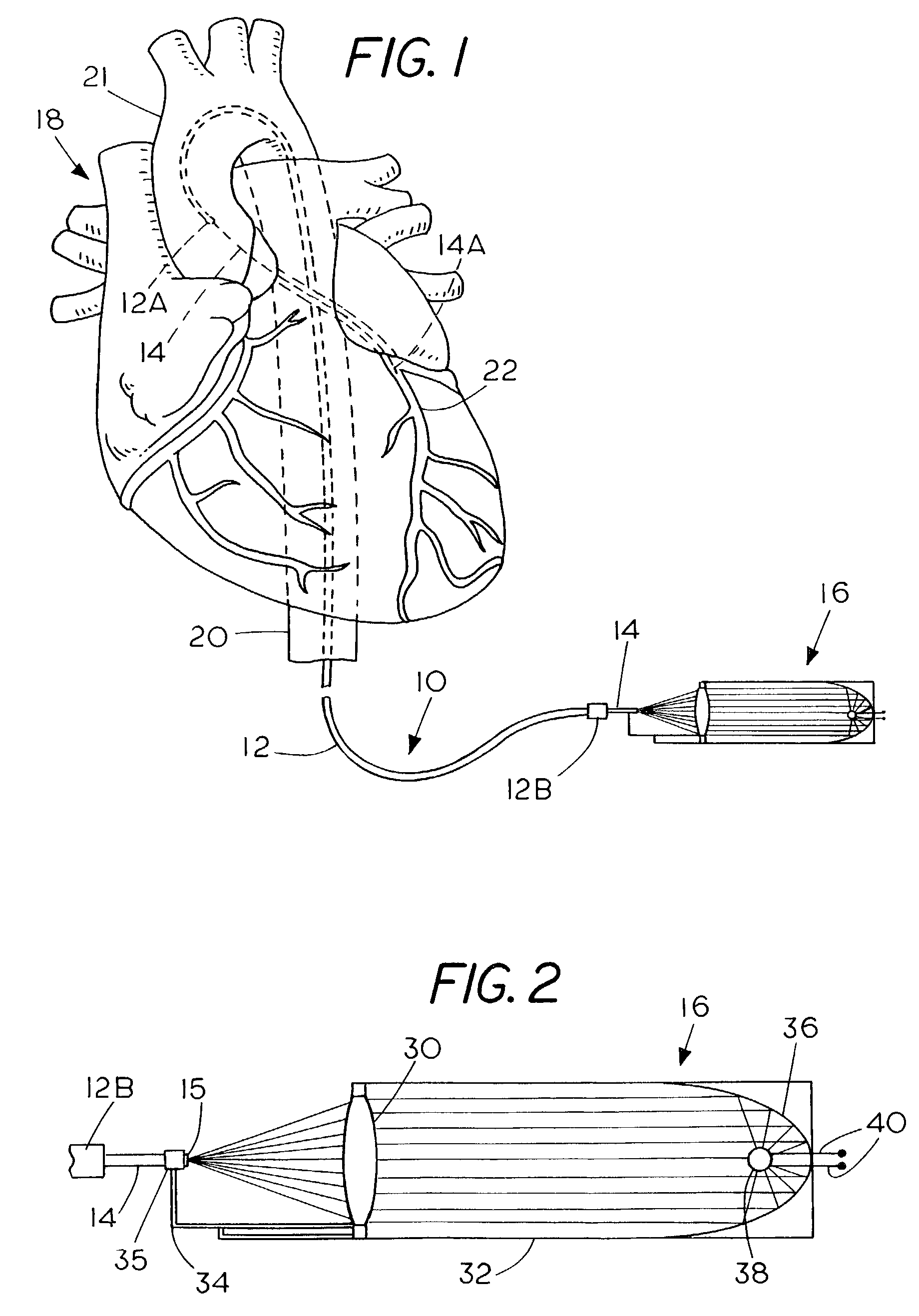
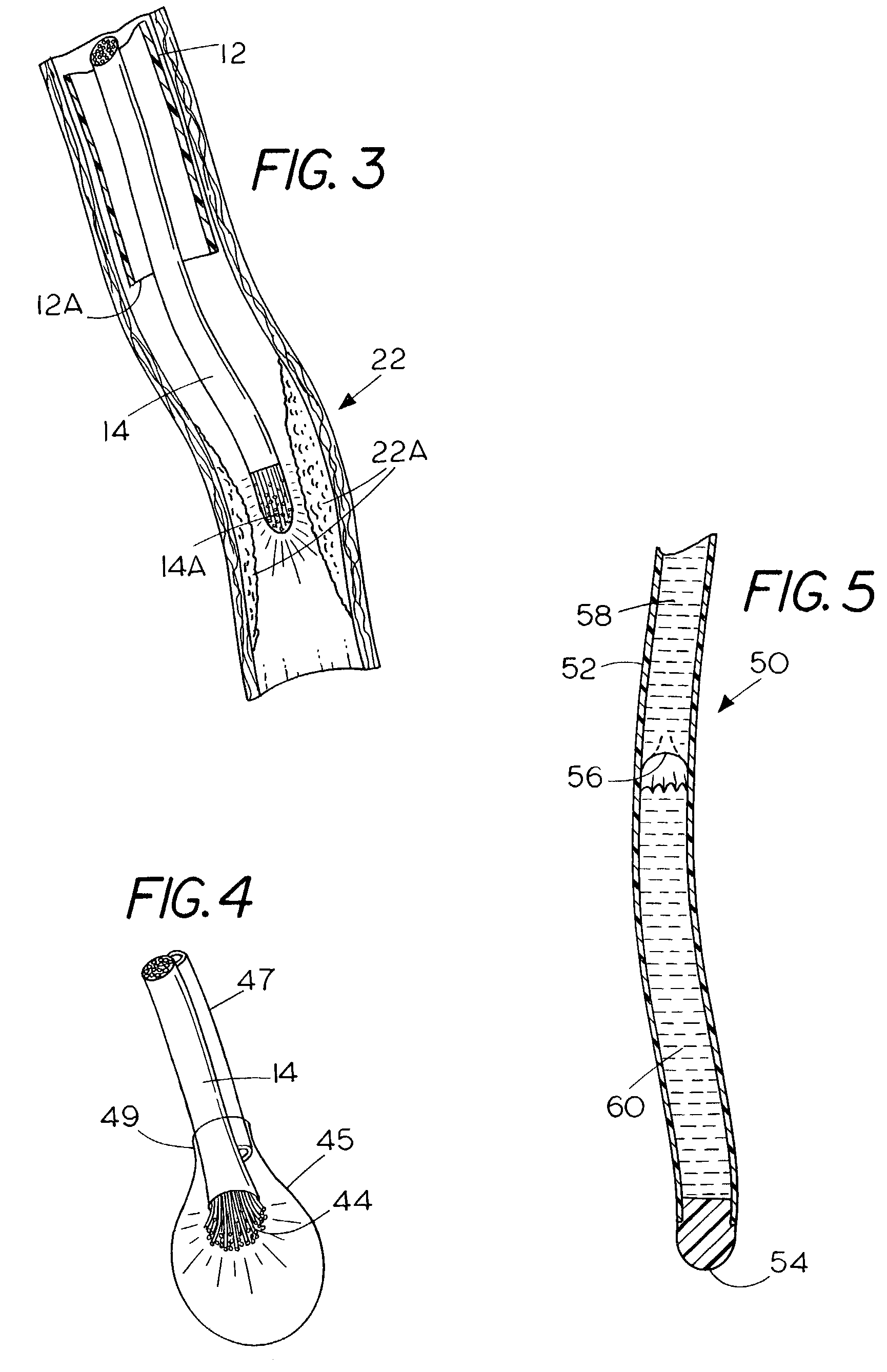
Apparatus and method for treating atherosclerotic vascular disease through light sterilization
InactiveUS7107996B2Reducing and eliminating bacterial infectionReduce inflammationTeeth fillingDiagnosticsPathogenic microorganismCoronary artery disease
A method and apparatus for treating gum disease includes a light producing dental appliance that is accessible exteriorly of the body for placement within the mouth of the patient to expose the mouth to light radiation of a selected wavelength and in an amount that is effective for killing or debilitating pathogenic microorganisms and especially Porphyromona gingivalis within the mouth of the patient such that the bacterial load carried to the heart is diminished thereby reducing or eliminating the symptoms of coronary artery disease, atherosclerosis vascular inflammation and plaque formation.
Owner:GANZ ROBERT A +1
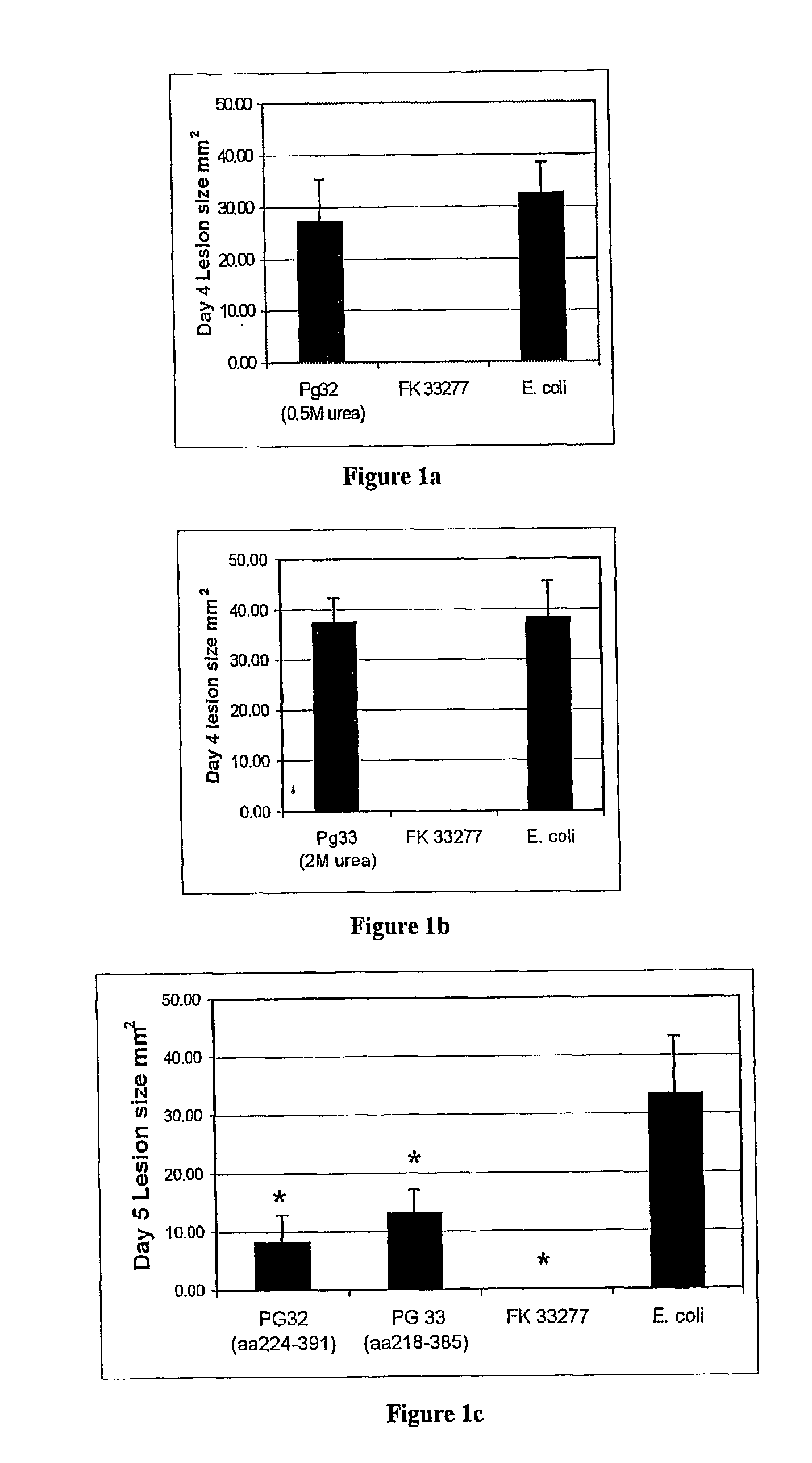
Porphyromonas gingivalis recombinant proteins and truncations
InactiveUS7204991B2Improve solubilityAntibacterial agentsCosmetic preparationsPorphyromonas gingivalisPolynucleotide
The present invention relates to soluble P. gingivalis polypeptides derived from PG32 and PG33 and to polynucleotides encoding these polypeptides. The P. gingivalis polypeptides and nucleotides can be used in compositions for use in raising an immune response in a subject against P. gingivalis and treating or preventing or reducing the severity of the condition known as periodontitis or in other conditions related to infection with P. gingivalis.
Owner:THE UNIV OF MELBOURNE EQUAL SHARE
P. gingivalis antigenic composition
The present invention provides an antigenic composition, the composition comprising at least one recombinant protein. The recombinant protein comprises at least one epitope. The epitope is reactive with an antibody which is reactive with a polypeptide having the sequence set out in SEQ. ID. NO. 3 or SEQ. ID. NO. 5. The invention also provides methods and compositions for the production of the recombinant protein. Also provided are methods for the diagnosis, treatment and prevention of P. gingivalis infection.
Owner:CSL LTD +1
Substituted benzoquinones and hydroquinones in the treatment of periodontal diseases
InactiveUS20100310478A1Reduce riskPrevent and reduce riskCosmetic preparationsOrganic chemistryBiofilmPorphyromonas gingivalis
An alkyl- or halo-substituted benzoquinone, hydroquinone or mixture thereof, for use in the treatment of a periodontal disease, and / or for use in the treatment of a condition which is caused by, transmitted by or exacerbated by P. gingivalis or by biofilm formation by P. gingivalis.
Owner:SYNTOPIX
Synthetic peptides containing protective epitopes for the treatment and prevention of periodontitis associated with porphyromonas gingivalis
InactiveUS6962706B1Mitigate prospectEfficient inductionCosmetic preparationsImpression capsEpitopePorphyromonas gingivalis
This invention relates to a peptide selected from the group: FLLDADHNTFGSVIPATGPLFTGTASS LYSANFESLIPANADPVVTTQNIIVTG LYSANFEYLIPANADPVVTTQNIIVTG TNPEPASGKMWIAGDGGNQP RYDDFTFEAGKKYTFTMRRAGMGDGTD DDYVFEAGKKYHFLLLMKKMGSGDGTE TNPEPASGKMWIAGDGGNQPARYDDFTFEAGKKYTFTMRRAGMGDGTD NTFGSVIPATGPL PASGKMWIAGDG EAGKKYTFTMRRA EAGKKYHFLMKKM. It also relates to compositions and use of these peptides for treating and testing Porphyromonas gingialis.
Owner:UNIVERSITY OF MELBOURNE +2
Oral antibacterial composition containing nisin and polylysine and preparation method thereof
InactiveCN105326656AImprove the bactericidal effectNo adverse reactionAntibacterial agentsCosmetic preparationsSide effectPorphyromonas gingivalis
The invention provides an oral antibacterial composition containing nisin and polylysine. According to the composition, the growth of oral pathogenic bacteria such as porphyromonas gingivalis, actinobacillus actinomycetemcomitans, streptococcus mutans, lactic acid bacillus, staphylococcus aureus and candida albicans can be effectively inhibited, and tooth decay, gingivitis, periodontitis, ozostomia and the like can be prevented; the composition can be prepared into toothpaste, mouth wash, refreshing and tasting chips and chewing gum which are used for preventing and treating oral diseases. Meanwhile, the invention provides a preparation method of the compositions such as the toothpaste, the mouth wash, the refreshing and tasting chips and the chewing gum. The oral antibacterial composition prepared through the method can prevent and treat various oral diseases and has no toxic and side effect and allergy or stimulation phenomenon on a human body.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Synthetic peptide constructs for the diagnosis and treatment of periodontitis associated with Porphyromonas gingivalis
InactiveUS7262271B2Prevent diseaseReducing colonisationAntibacterial agentsCosmetic preparationsBacteroidesPorphyromonas gingivalis
The present invention relates to an oral composition and an immunogenic composition for the suppression of the pathogenic effects of the intra-oral bacterium Porphyromonas gingivalis associated with periodontal disease.
Owner:UNIVERSITY OF MELBOURNE
Porphorymonas gingivalis polypeptides and nucleotides
InactiveUS7544777B2Rapid and easy identificationImproving immunogenicityCosmetic preparationsBacteriaPorphyromonas gingivalisNucleotide
Owner:CSL LTD +1
Porphyromonas Gingivalis Polypeptides Useful in the Prevention of Periodontal Disease
The invention is directed to vaccine compositions and methods based on P. gingivalis proteins identified to be regulated by haem availability that can be used in the prevention and treatment of periodontal disease. In particular, two specific internalin-like P. gingivalis proteins, namely PG0350 and PG1374 involved in the internalization of P. gingivalis by host cells, the hypothetical protein, PG1019 purported to be a cell surface lipoprotein and the alkyl hydroperoxide reductase protein, PG0618 have been identified as useful targets for the prevention and treatment of periodontal disease.
Owner:ORAL HEALTH AUSTRALIA PTY LTD
Porphyromonas gingivalis polypeptides and nucleotides
InactiveUS20100034908A1Enhance immune responseAntibacterial agentsBiocidePorphyromonas gingivalisBiology
The present invention relates to isolated Porphyromonas gingivalis polypeptides and nucleotides. The polypeptides include an amino acid sequence selected from the group consisting of: SEQ. ID. NO. 110; SEQ. ID. NO. 111; SEQ. ID. NO. 112; SEQ. ID. NO. 113; SEQ ID NO: 120; SEQ. ID. NO. 123; SEQ. ID. NO. 124; SEQ. ID. NO. 125; SEQ. ID. NO. 130; SEQ. ID. NO. 131; SEQ. ID. NO. 132; SEQ. ID. NO. 133; SEQ. ID. NO.135; SEQ. ID. NO. 136; SEQ. ID. NO. 137; SEQ. ID. NO. 138; SEQ. ID. NO. 143; SEQ. ID. NO. 144; SEQ. ID. NO. 145; SEQ. ID. NO. 146; SEQ. ID. NO. 147; SEQ. ID. NO. 148; and amino acid sequences at least 95% identical thereto.
Owner:CSL LTD +1
Compound bactericide for efficient degradation of landscaping garbage and preparation method thereof
InactiveCN101988040ARealize the transformation of resourcesZero pollutionFungiBacteriaPorphyromonas gingivalisAspergillus
The invention relates to a compound bactericide for efficient degradation of landscaping garbage and a preparation method thereof. The compound bactericide mainly consists of strains and a carrier, wherein the strains comprise the following components in parts by weight: 15-30 parts of fibre porphyromonas gingivalis, 10-25 parts of basidiomycetes, 5-15 parts of bacillus, 15-35 parts of aspergillus, 5-35 parts of neurospora, 8-20 parts of trichoderma, 15-20 parts of actinomycetes and 5-15 parts of saccharomycetes. The compound bactericide is fully mixed with the landscaping garbage in a fermentation device and is synergized through different bactericides under the aerobic condition, thereby the landscaping garbage degradation efficiency is improved, and the organic matter is rapidly corrupted and converted into green organic fertilizer capable of improving soil. The application of the compound bactericide for efficient degradation of landscaping garbage can effectively solve the problems of land occupation caused by land filling or pollution to the atmospheric environment caused by burning, and the like.
Owner:NANKAI UNIV +1
P. gingivalis antigenic composition
The present invention provides an antigenic composition, the composition comprising at least one recombinant protein. The recombinant protein comprises at least one epitope. The epitope is reactive with an antibody which is reactive with a polypeptide having the sequence set out in SEQ. ID. NO. 3 or SEQ. ID. NO. 5. The invention also provides methods and compositions for the production of the recombinant protein. Also provided are methods for the diagnosis, treatment and prevention of P. gingivalis infection.
Owner:CSL LTD +1
Identification of Porphyromonas gingivalis virulence polynucleotides for diagnosis, treatment, and monitoring of periodontal diseases
ActiveUS20060078950A1Reduce the amount of solutionAntibacterial agentsCosmetic preparationsPeriodontal diseasePorphyromonas gingivalis
The invention provides compositions and methods for the detection of Porphyromonas gingivalis and for the treatment and prevention of diseases and infections caused by P. gingivalis.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Antigenic complex for the diagnosis and treatment of porphyromonas gingivalis infection
ActiveUS20110081358A1Induce immune responseAntibacterial agentsBacterial antigen ingredientsAntigenPorphyromonas gingivalis
Owner:UNIVERSITY OF MELBOURNE
Immunology Treatment for Biofilms
The invention provides a composition for use in raising an immune response to P. gingivalis in a subject, the composition comprising an amount effective to raise an immune response of at least one polypeptide having an amino acid sequence substantially identical to at least 50 amino acids, or an antigenic or immunogenic portion, of one of the polypeptides corresponding to accession numbers selected from the group consisting of AAQ65462, AAQ65742, AAQ66991, AAQ65561, AAQ66831, AAQ66797, AAQ66469, AAQ66587, AAQ66654, AAQ66977, AAQ65797, AAQ65867, AAQ65868, AAQ65416, AAQ65449, AAQ66051, AAQ66377, AAQ66444, AAQ66538, AAQ67117 and AAQ67118. The invention also provides a method of preventing or treating a subject for P. gingivalis infection comprising administering to the subject a composition of the invention
Owner:ORAL HEALTH AUSTRALIA PTY LTD
Diagnostics and treatments of periodontal disease
InactiveUS20110213129A1Mitigate prospectEfficient inductionCosmetic preparationsOrganic active ingredientsPorphyromonas gingivalisArginine
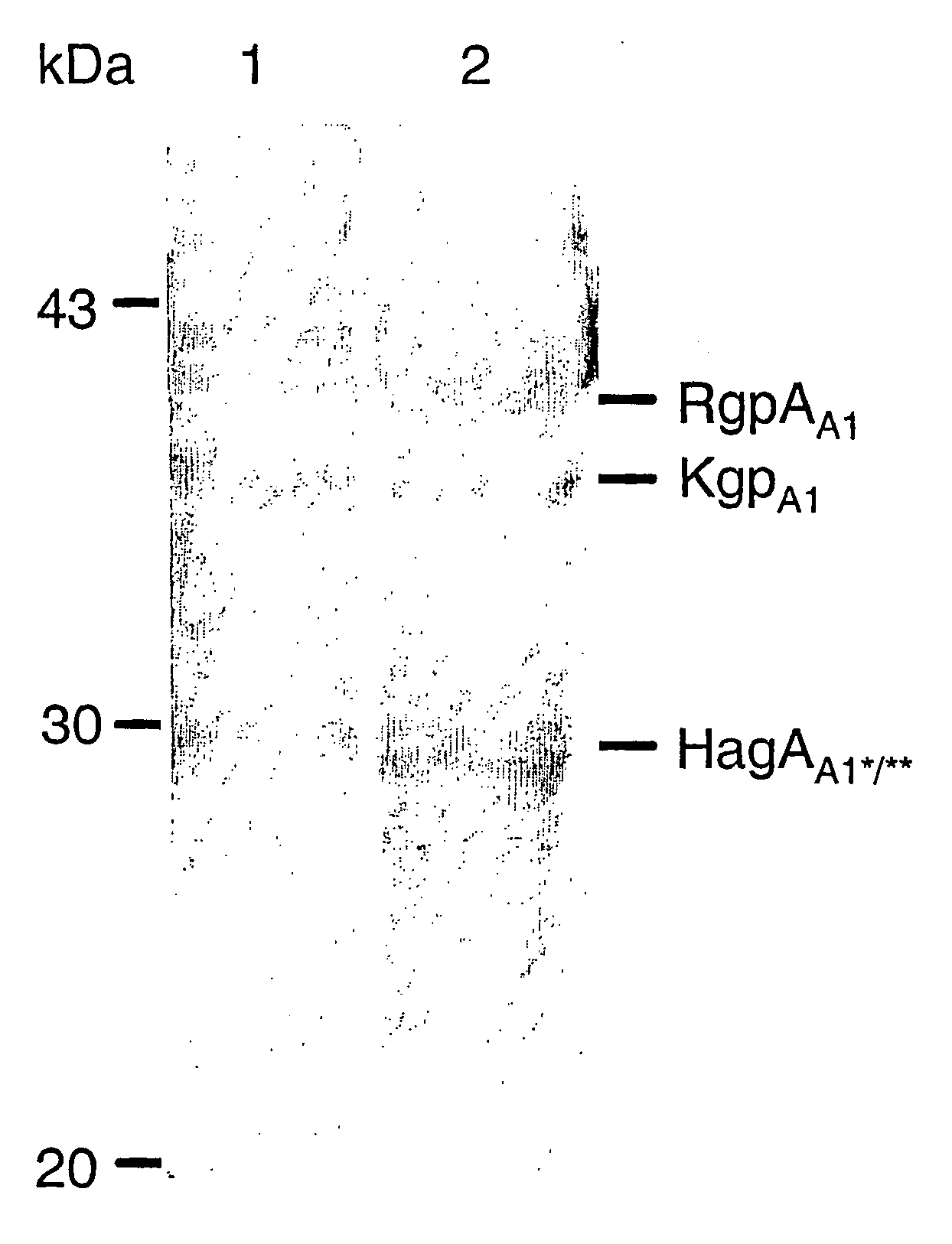
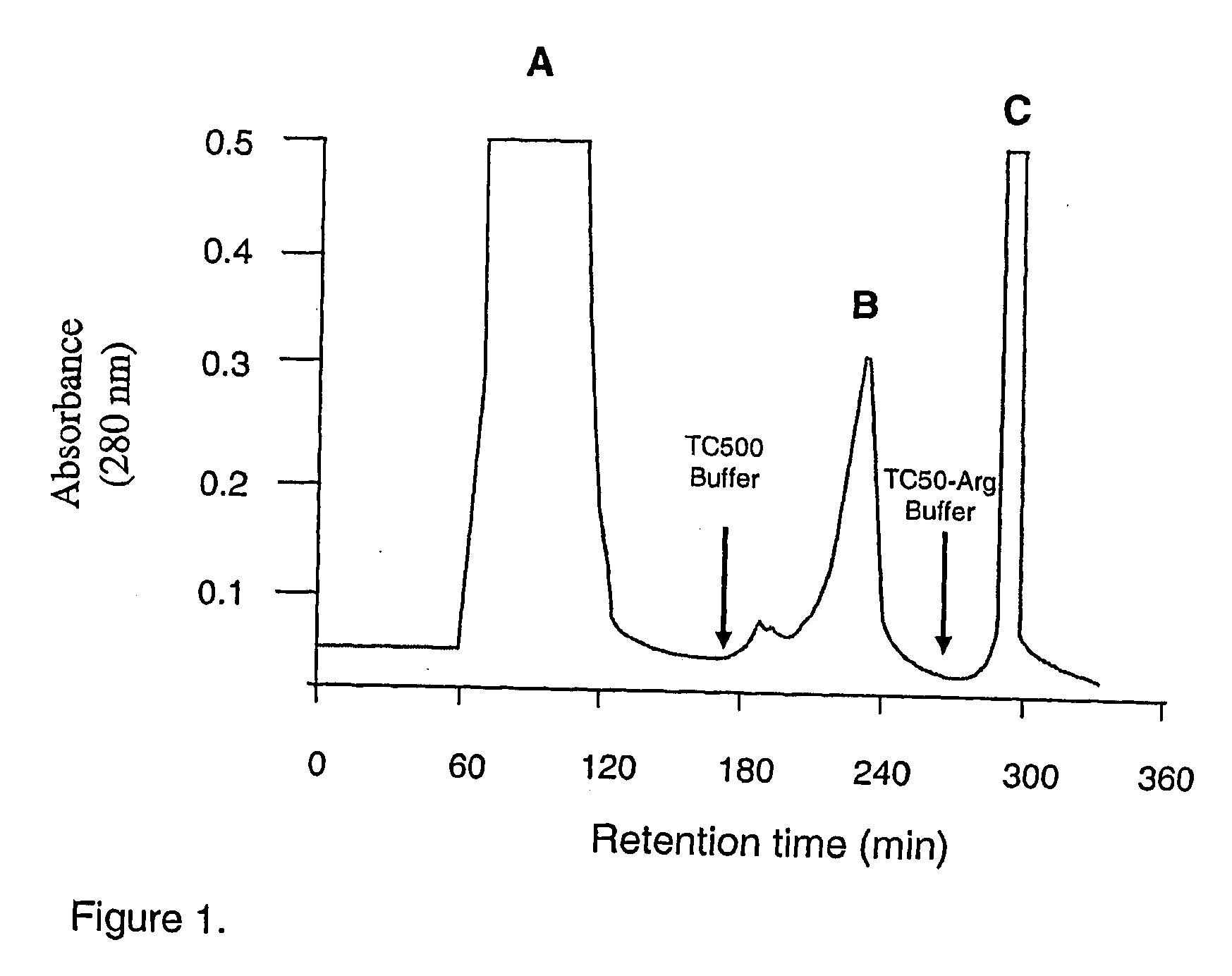
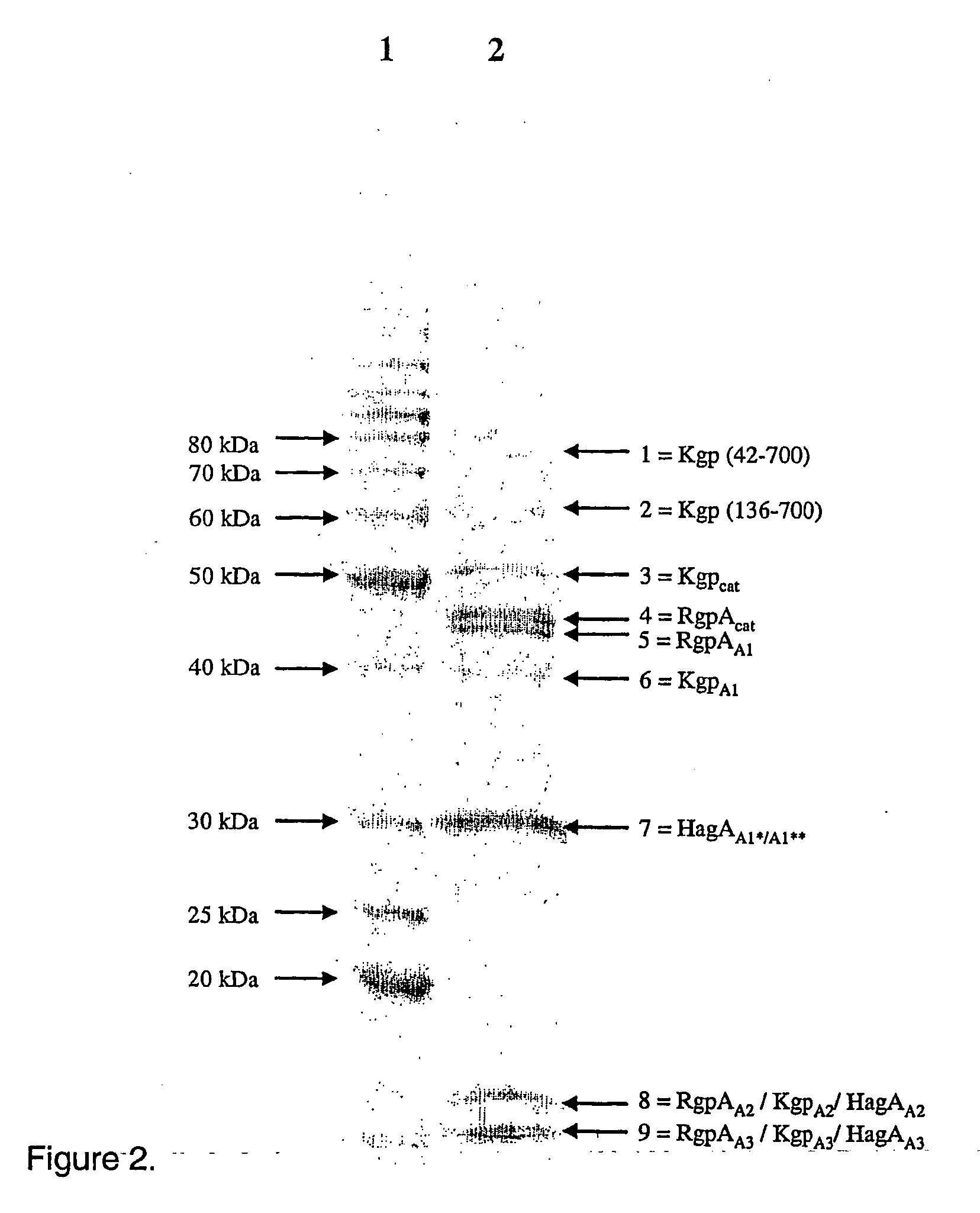
This invention relates to the PrtR-PrtK cell surface protein of Porphyromonas gingivalis and in particular a multimeric cell association protein complex comprising the PrtR and PrtK proteins. Accordingly the invention provides a substantially purified antigenic complex for use in raising an antibody response directed against Porphyromonas gingivalis. The complex comprises at least one multimeric protein complex of arginine-specific and lysine-specific thiol endopeptidases each containing at least one adhesin domain, the complex having a molecular weight of greater than about 200 kDa. The invention also relates to pharmaceutical compositions and associated agents based on said complex for the detection, prevention and treatment of Periodontal disease associated with P. gingivalis.
Owner:UNIVERSITY OF MELBOURNE
Multiple PCR fast detecting method for oral cavity pathogen
InactiveCN101270381ASame reaction conditionsSensitive methodMicrobiological testing/measurementPorphyromonas gingivalisElectrophoresis
The invention relates to a multiple PCR rapid detection method of common pathogen in mouth such as Actinobacillus actinomycetemcomitans, Bacterides forsythus, F. nucleatum and Porphyromones gingivalis and belongs to the field of molecular biology technology. The invention utilizes the multiple PCR to increase specific gene of Actinobacillus actinomycetemcomitans, Bacterides forsythus, F. nucleatum and Porphyromones gingivalis in mouth and utilizes electrophoresis to detect the specific gene. By designing the primers of Actinobacillus actinomycetemcomitans, Bacterides forsythus, F. nucleatum and Porphyromones gingivalis, the multiple PCR rapid detection method has the advantages of the uniform reaction condition, the sensitivity and the strong specificity of the method, the simultaneous detection and identification of the four bacteria in the specimen, accurate judgment of infection of different bacteria and greatly promoting the detection speed and the detection efficiency.
Owner:肖水清
Methods and compositions for prevention of angioproliferation
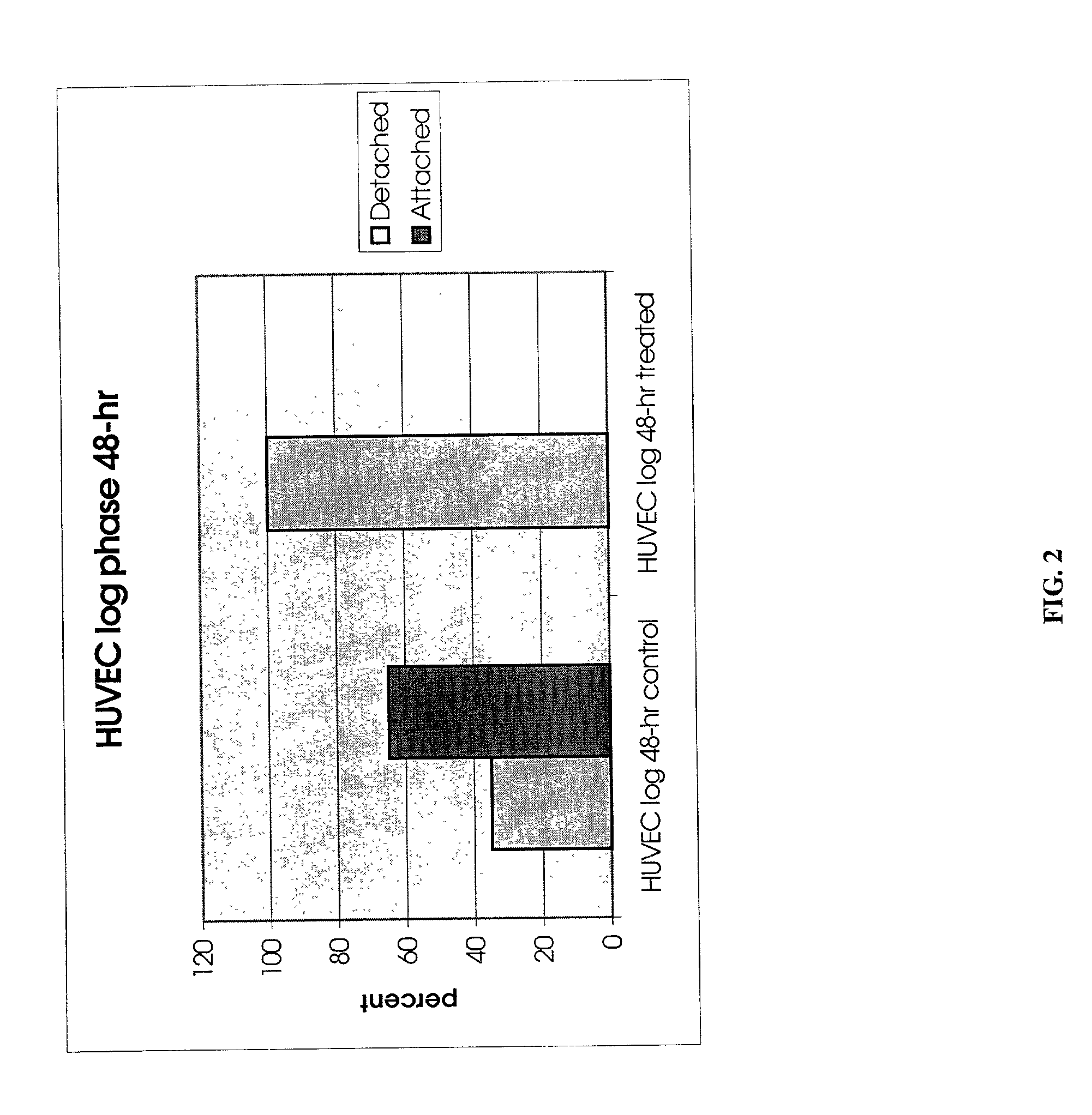
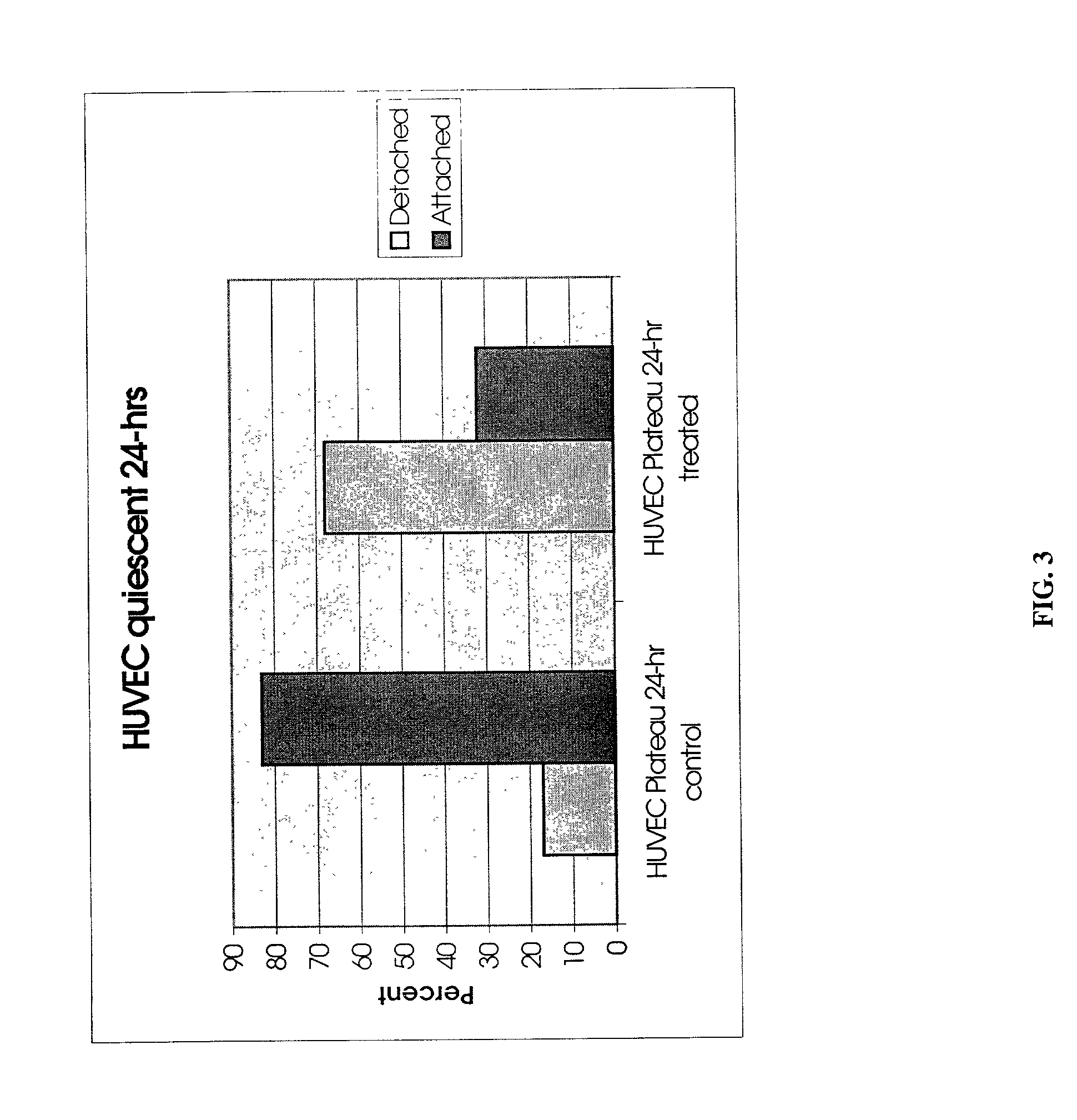
InactiveUS20050019318A1Inhibition formationPeptide/protein ingredientsHydrolasesHemagglutininProteinase activity
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Biofilm treatment
The invention provides a method of preventing, inhibiting or reducing a P. gingivalis biofilm in a subject comprising administering to the subject a pharmaceutical composition comprising an inhibiting agent of a polypeptide that reduces or inhibits biofilm formation and / or biofilm development. Also provided are compositions useful in the prevention, inhibition or treatment of periodontal disease or P. gingivalis infection.
Owner:ORAL HEALTH AUSTRALIA PTY LTD
Biofilm Treatment
The invention provides a method of preventing, inhibiting or reducing a P. gingivalis biofilm in a subject comprising administering to the subject a pharmaceutical composition comprising an inhibiting agent of a polypeptide that reduces or inhibits biofilm formation and / or biofilm development. Also provided are compositions useful in the prevention, inhibition or treatment of periodontal disease or P. gingivalis infection.
Owner:ORAL HEALTH AUSTRALIA PTY LTD
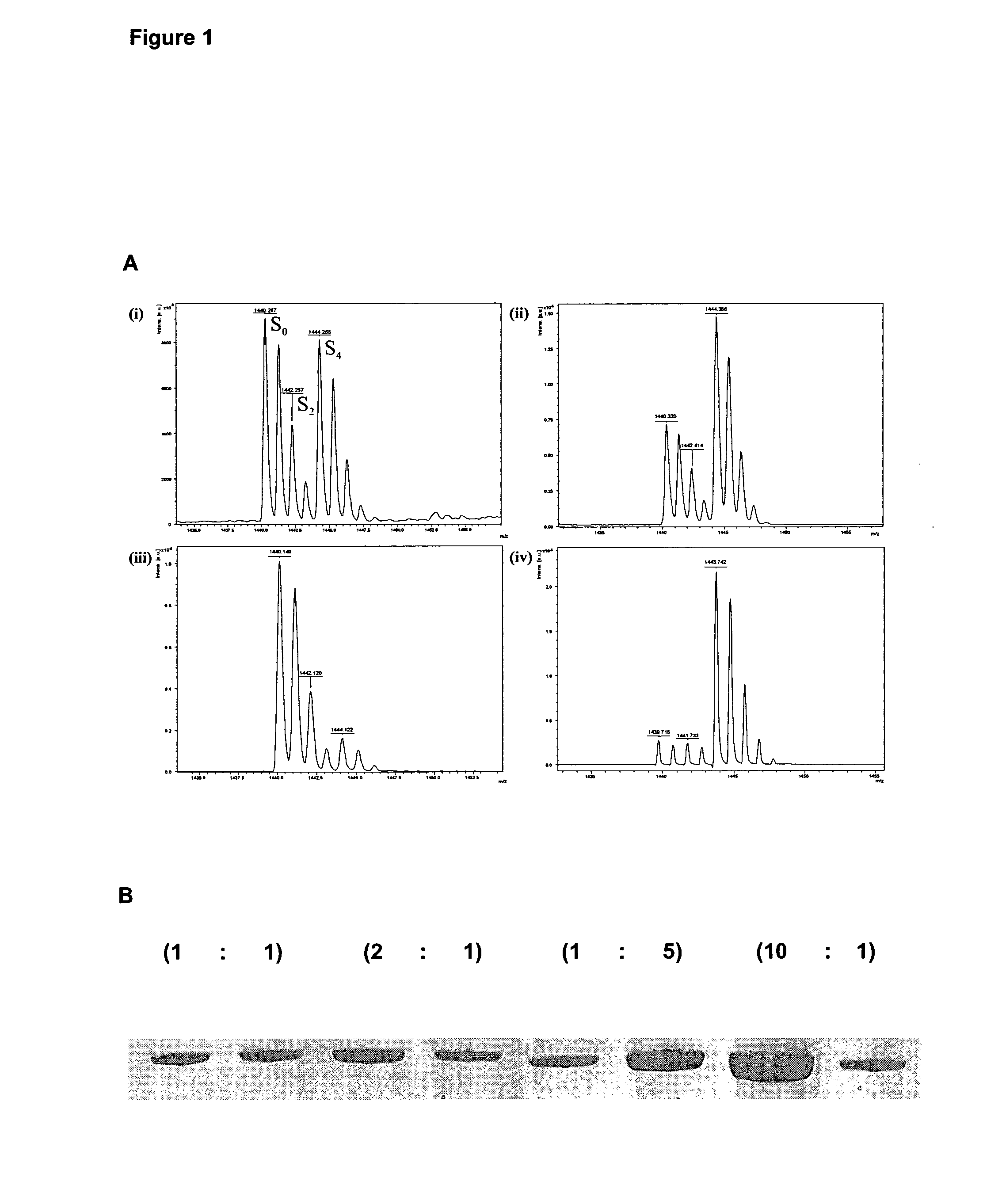
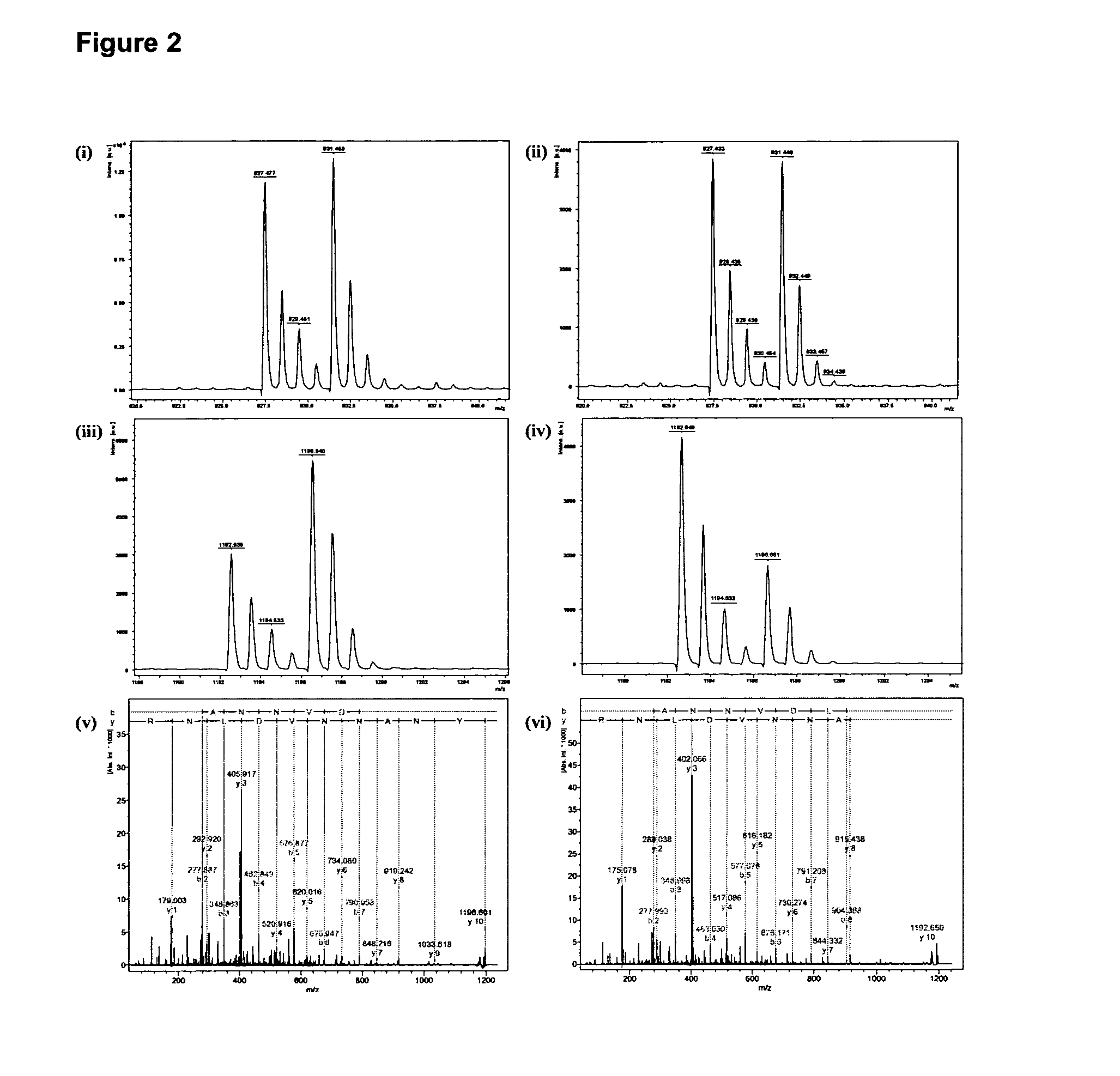
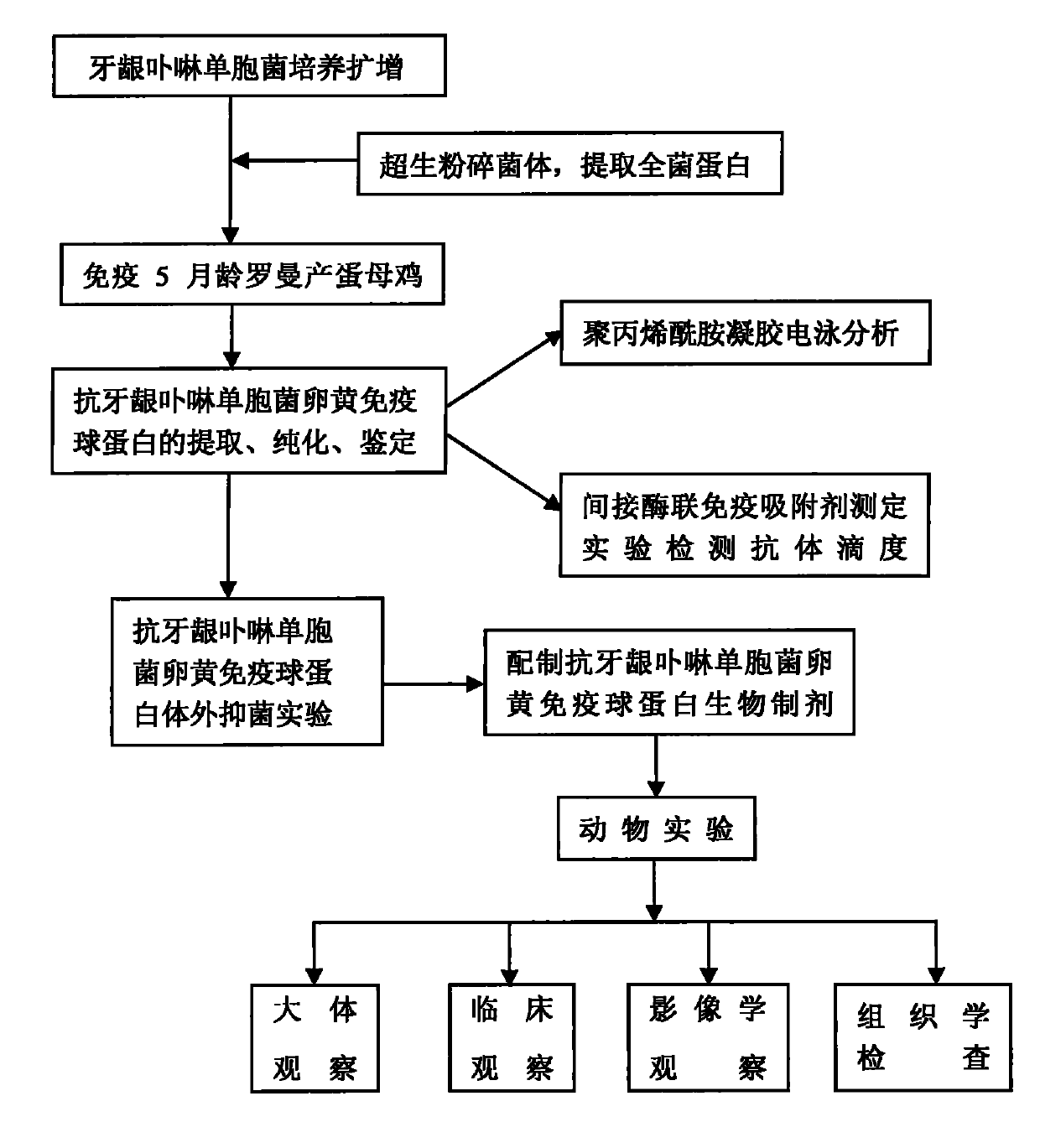
Method for preparing yolk immunoglobulin vaccine for resisting porphyromonas gingivalis
ActiveCN101791405ALow priceReduce manufacturing costAntibacterial agentsBacterial antigen ingredientsYolkMycoprotein
The invention relates to a method for preparing a yolk immunoglobulin vaccine for resisting porphyromonas gingivalis, which comprises the following operating steps of: (1) culturing and separating an international standard strain of the porphyromonas gingivalis to obtain the porphyromonas gingivalis; (2) obtaining mycoprotein of the porphyromonas gingivalis; (3) mixing a whole bacterium soluble protein antigen of the porphyromonas gingivalis with a freunds incomplete adjuvant uniformly to prepare an intravenous injection preparation, performing an intravenous injection under a chicken wing of a laying hen, and collecting eggs laid by the laying hen; and (4) extracting yolk immunoglobulin for resisting the porphyromonas gingivalis in the eggs. The method has a low production cost and a high yield because 100 milligrams of the yolk immunoglobulin can be obtained from one egg; and the method is simple, convenient and quick to operate and has the advantages of heat resistance, acid resistance, good stability and the like. Under an acid condition of which the temperature is not over 75 DEG C and the pH is more than 4, the yolk immunoglobulin vaccine can still well maintain the biological activity, can be stored for about 3 months at the normal temperature, and can be stored for 6 to 12 months at 4 DEG C with non-decreased antibody activity.
Owner:ANHUI MEDICAL UNIV
Porphyromonas gingivalis polypeptides useful in the prevention of periodontal disease
InactiveUS20130236488A1Bacterial antigen ingredientsPeptide/protein ingredientsPorphyromonas gingivalisHeme
The invention is directed to vaccine compositions and methods based on P. gingivalis proteins identified to be regulated by haem availability that can be used in the prevention and treatment of periodontal disease. In particular, two specific internalin-like P. gingivalis proteins, namely PG0350 and PG1374 involved in the internalization of P. gingivalis by host cells, the hypothetical protein, PG1019 purported to be a cell surface lipoprotein and the alkyl hydroperoxide reductase protein, PG0618 have been identified as useful targets for the prevention and treatment of periodontal disease.
Owner:ORAL HEALTH AUSTRALIA PTY LTD
Diagnostics and treatments of Periodontal disease
InactiveUS20070189982A1Mitigate prospectEfficient inductionCosmetic preparationsOrganic active ingredientsPorphyromonas gingivalisArginine
This invention relates to the PrtR-PrtK cell surface protein of Porphyromonas gingivalis and in particular a multimeric cell association protein complex comprising the PrtR and PrtK proteins. Accordingly the invention provides a substantially purified antigenic complex for use in raising an antibody response directed against Porphyromonas gingivalis. The complex comprises at least one multimeric protein complex of arginine-specific and lysine-specific thiol endopeptidases each containing at least one adhesin domain, the complex having a molecular weight of greater than about 200 kDa. The invention also relates to pharmaceutical compositions and associated agents based on said complex for the detection, prevention and treatment of Periodontal disease associated with P. gingivalis.
Owner:UNIVERSITY OF MELBOURNE
Methods and compositions for angioproliferative disorder treatment
InactiveUS20020192206A1Prevent implantationPeptide/protein ingredientsGenetic material ingredientsAbnormal tissue growthPorphyromonas gingivalis
An invention is provided whereby methods and compositions having angiostatic activity are utilized to treat angioproliferative disorders, to prevent conception, and to treat a wide variety of pathologies in which it is desirable to limit the production of new vasculature. Specifically, compositions containing proteinases derived from the pathogen Porphyromonas gingivalis capable of treating cancer through disruption of cell-cell and cell-matrix adhesion bonds associated with malignant tumor proliferation are disclosed.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Identification of Porphyromonas gingivalis virulence polynucleotides for diagnosis, treatment, and monitoring of periodontal diseases
ActiveUS7416852B2Antibacterial agentsNervous disorderVirulent characteristicsPorphyromonas gingivalis
The invention provides compositions and methods for the detection of Porphyromonas gingivalis and for the treatment and prevention of diseases and infections caused by P. gingivalis.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Antigenic Complex for the Diagnosis and Treatment of Porphyromonas Gingivalis Infection
InactiveUS20090169568A1Induce immune responseAntibacterial agentsBacterial antigen ingredientsAntigenPorphyromonas gingivalis
Owner:UNIVERSITY OF MELBOURNE
Methods of use for therapeutics targeting the pathogen porphyromonas gingivalis
InactiveUS20170014468A1Reducing circulating activityEnhance phagocytosisDipeptide ingredientsAmide active ingredientsPorphyromonas gingivalisArthritis
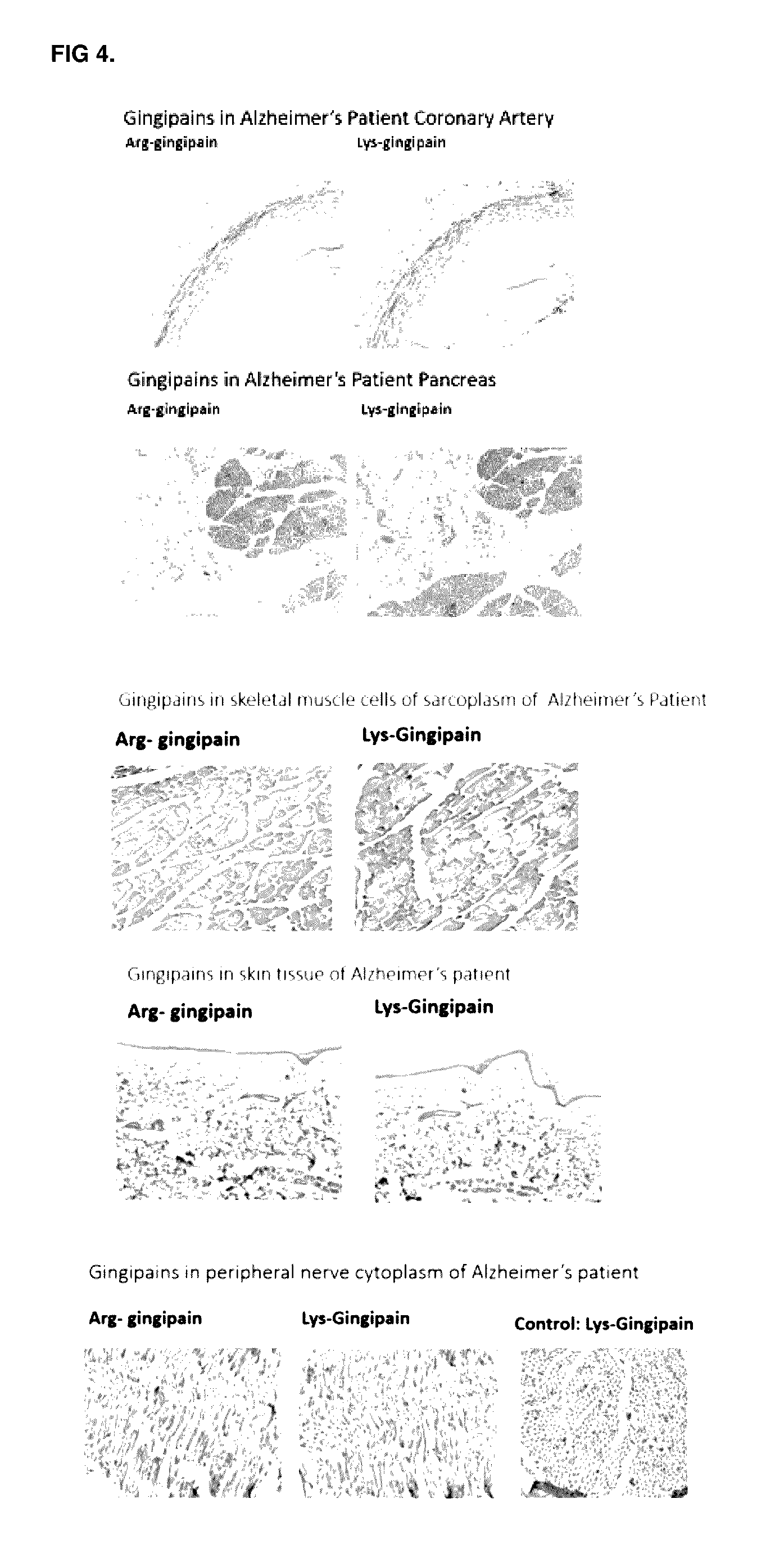
The invention relates to compositions, formulations, vaccines and antibodies for the prevention and / or treatment of aging and brain disorders, including Alzheimer's disease, diabetes, cardiovascular disease, retinal disorders and arthritis. The invention also provides methods of treatment of aging disorders, brain disorders, including Alzheimer's disease, diabetes, cardiovascular disease, retinal disorders, and arthritis by administering compositions, formulations, vaccines and antibodies described in the specification. The invention further provides methods for diagnosing or assessing risk of development of brain disorders in humans and animals. The invention further provides animal models for testing novel therapeutics for brain disorders.
Owner:CORTEXYME INC
Porphyromonas gingivalis 1435/1449 LPS as an immune modulator
InactiveUS7622128B2Cosmetic preparationsBacterial antigen ingredientsAutoimmune conditionPorphyromonas gingivalis
Owner:UNIV OF WASHINGTON
Application of Antimicrobial and Glycemic Control Activities of Lo Han Kuo Fruit (Siraitia grosvenorii)
InactiveUS20120141386A1Prevention of dental cariesGreat market potentialAntibacterial agentsCosmetic preparationsPorphyromonas gingivalisMonilinia laxa
The present invention discloses an application of the extract of Lo Han Kuo (LHK) fruit (Siraitia grosvenorii Swingle) for its dual related functions. Specifically, this is related to the use of LHK extract for its antimicrobial activities against pathogens Streptococcus mutans, Porphyromonas gingivalis, A. actinomycecomitan, F. nucleatum, and Candida albicans, and its low glycemic index for use by general population as well as the diabetics to improve oral health.
Owner:ORACEUTICALS
Porphorymonas gingivalis polypeptides and nucleotides
InactiveUS20070189981A1Rapid and easy identificationImproving immunogenicityCosmetic preparationsBacteriaPorphyromonas gingivalisBiology
The present invention relates to isolated Porphorymonas gingivalis polypeptides and nucleotides. The polypeptides include; an amino acid sequence selected from the group consisting of SEQ. ID. NO. 265 to SEQ. ID. NO. 528, SEQ. ID. NO. 531 and SEQ. ID. NO. 532; or an amino acid sequence at least 85%, preferably at least 95%, identical to an amino acid sequence selected from the group consisting of SEQ. ID. NO. 265 to SEQ. ID. NO. 528, SEQ. ID. NO. 531 and SEQ. ID. NO. 532; or at least 40 amino acids having a contiguous sequence of at least 40 amino acids identical to a contiguous amino acid sequence selected from the group consisting of SEQ. ID. NO. 265 to SEQ. ID. NO. 528, SEQ. ID. NO. 531 and SEQ. ID. NO. 532.
Owner:CSL LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com