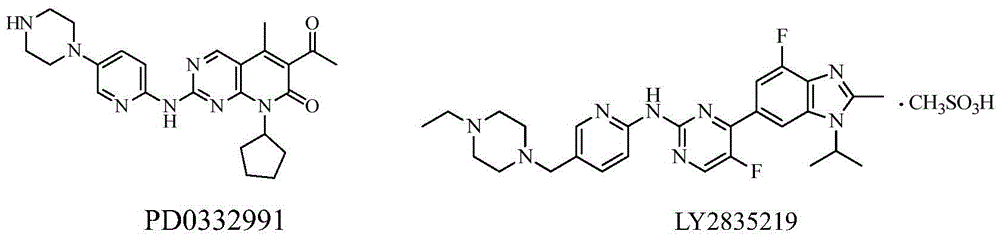
CDK kinase inhibitor
A solvate, selected technology, used in medical preparations containing active ingredients, organic active ingredients, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
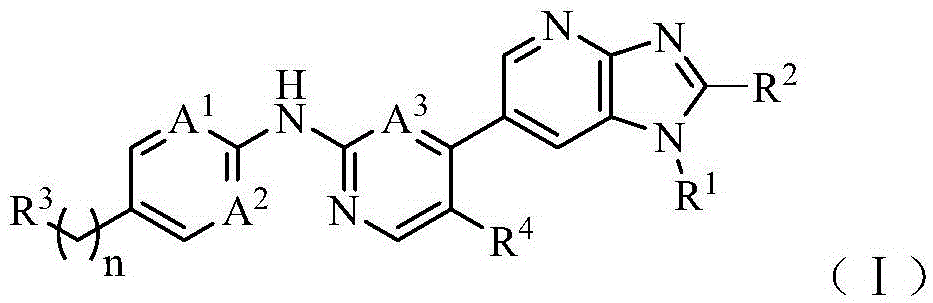
[0056] The present invention also provides a preparation method for the compound of formula (I), which includes but is not limited to the following process route (wherein, the definitions represented by each abbreviation are as follows: DCM: dichloromethane; DIPEA: N,N-diisopropyl Ethylamine; DMSO: dimethyl sulfoxide; EA: ethyl acetate; HATU: 2-(7-azobenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate Esters; MeOH: methanol; NBS: N-bromosuccinimide; PE: petroleum ether; THF: tetrahydrofuran; Xant-phos: 4,5-bisdiphenylphosphine-9,9-dimethyloxa anthracene; x-phos: 2-dicyclohexylphosphine-2',4',6'-triisopropylbiphenyl):
[0057] Routing:
[0058]
[0059] R 1 , R 2 , R 3 , R 4 , n, A 1 、A 2 、A 3 As mentioned above, X represents a halogen selected from fluorine, chlorine, bromine and iodine.
[0060] The specific exemplary steps are as follows:
[0061] 1. Preparation of Intermediate 1
[0062] Dissolve raw material 1 and organic base in an organic solven...
experiment example 1
[0090] Experimental Example 1 The in vitro enzymatic activity test of the compound of the present invention
[0091] Test substance: some compounds 1 and 3 of the present invention, see the preparation examples of each compound for their chemical names and preparation methods.
[0092] Control drug: LY2835219, see the background technology section for the structural formula, purchased from Wuhan Yongcan Biotechnology Co., Ltd. The meanings represented by the abbreviations in the following experiments are as follows:
[0093]
[0094] Experimental method: Determination of inhibitory activity of CDK kinases by CaliperMobilityShift method
[0095] 1.1x kinase buffer preparation:
[0096] 1) Preparation of 1x CDK4 / 9 kinase buffer
[0097] Take HEPES with a mother liquor concentration of 1000mM at pH 7.5 and TritonX-100 with a mother liquor concentration of 10%, add ultrapure water and mix well so that the final concentration of HEPES is 20mM and the final concentration of Tr...
experiment example 2
[0126] In vitro cytological inhibitory activity of experimental example 2 compounds of the present invention
[0127] Test substance: some compounds 1 and 3 of the present invention, see the preparation examples of each compound for their chemical names and preparation methods.
[0128] Control drug: LY2835219, see the background technology section for the structural formula, purchased from Wuhan Yongcan Biotechnology Co., Ltd.
[0129] The meanings represented by the abbreviations of the following experiments are as follows:
[0130]
[0131] Experimental method: adopt BrdU method (BrdU cell proliferation test kit, CellSignalingTechnology company) to carry out cell proliferation detection
[0132] 1. Reagent and Compound Preparation
[0133] 1) 1 times lotion preparation:
[0134] Dilute the washing solution whose mother liquor concentration is 20 times to 1 times washing solution with ultrapure water.
[0135] 2) Preparation of 1-fold detection antibody solution:
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com