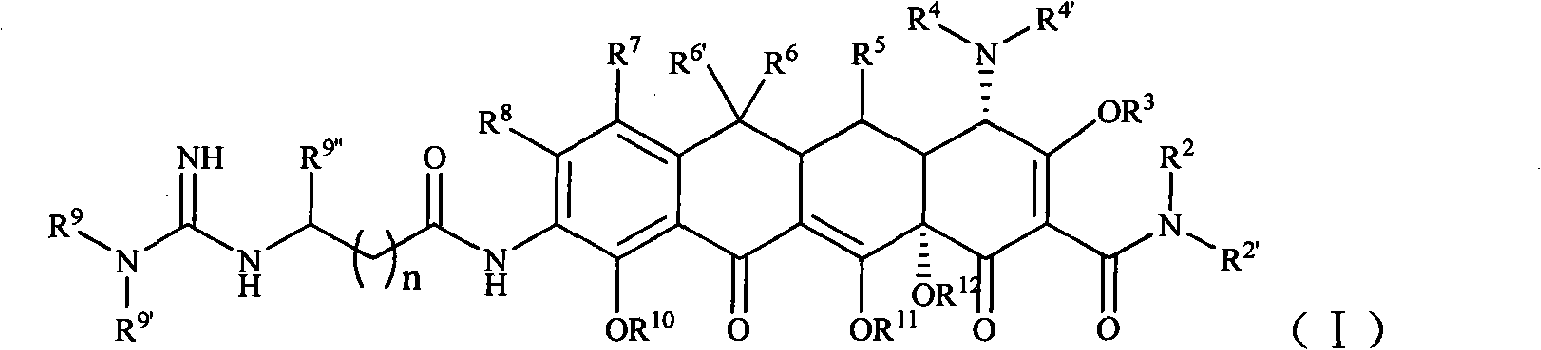
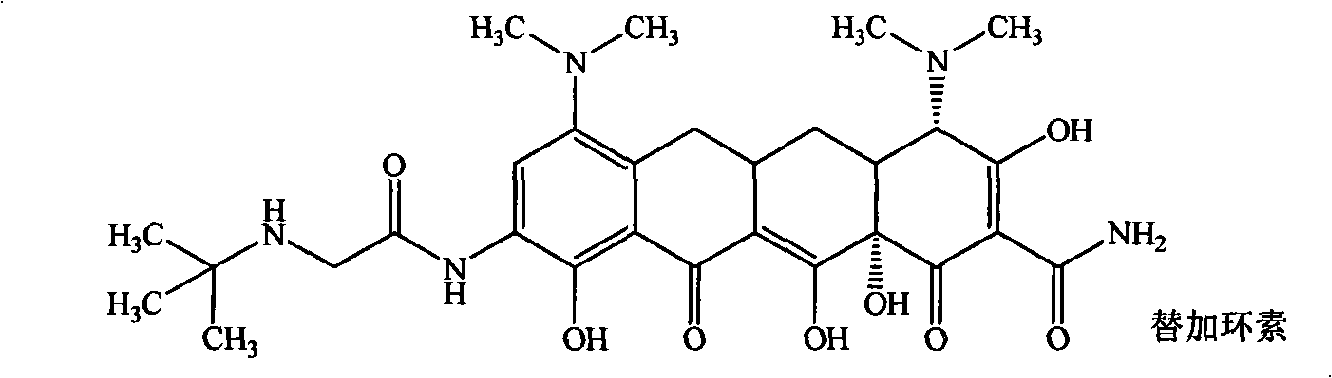
Guanidinoalkanoylamino substituted tetracycline derivatives
A technology based on alkylamine and sulfonyl groups, applied in the field of medicine, can solve problems such as inconvenient medication, unsatisfactory activity of Gram-negative bacteria, and pain of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Example 1 [S-(4α, 12aα)]-9-[2-[(N'-tert-butyl)guanidine]acetamido]-4,7-bis(dimethylamine base)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-tetracenecarboxamide (compound 1) Preparation
[0105] Step 1 [S-(4α,12aα)]-9-amino-4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10 , 12, 12a-4 Preparation of Hydroxy-1,11-dioxo-2-tetracenecarboxamide
[0106] Throw 11.4g (25mmol) [S-(4α,12aα)]-4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro in the reaction bottle -3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide dihydrochloride, dissolved in 80ml of concentrated sulfuric acid, stirred and cooled in an ice bath, then added 3.4g Sodium nitrate, and the mixture was stirred in an ice bath for 1 h. After the reaction was complete, the mixture was added dropwise to 1000 ml of ether, and a solid was precipitated, which was washed with a small amount of ether and dried. Add the solid to 50ml of ethanol, then add 1g of 10% pall...
Embodiment 2
[0115] Example 2 [S-(4α, 12aα)]-9-[2-[(N', N'-dimethyl)guanidino]acetamido]-4,7-bis(dimethylamine base)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-tetracenecarboxamide (compound 2) Preparation
[0116] The preparation method refers to Step 3 of Example 1, and puts [S-(4α, 12aα)]-9-[(chloroacetyl)amino]-4,7-bis(dimethylamino)-1,4,4a, 3.1g (5mmol) and 2.3 g (15 mmol) of N',N'-dimethylguanidine hydrochloride was reacted to obtain 2.4 g of the target compound, yield: 81.5%.
[0117] Molecular formula: C 28 h 37 N 7 o 8 Molecular weight: 599.64 Mass spectrum (m / e): 601 (M+1)
[0118] Elemental analysis: measured value: C, 55.84%; H, 6.48%; N, 16.11%;
[0119] Theoretical value: C, 56.08%; H, 6.22%; N, 16.35%;
[0120] 1 H-NMR (600MHz, CDCl 3 ): δ1.43(t, 1H), 1.75(t, 1H), 2.27(s, 1H), 2.31(s, 1H), 2.32(m, 1H), 2.35(t, 1H), 2.43(d, 1H), 2.44(s, 6H), 2.45(s, 6H), 2.66(d, 1H), 2.83(s, 6H), 3.16(d, 1H), 3.58(s, 2H), 5.16(s, 1H ), 5.41(s, 1H)...
Embodiment 3
[0121] Example 3 [S-(4α, 12aα)]-9-[2-[(N', N'-diethyl)guanidine]acetamido]-4,7-bis(dimethylamine base)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-tetracenecarboxamide (compound 3) Preparation
[0122] The preparation method refers to Step 3 of Example 1, and puts [S-(4α, 12aα)]-9-[(chloroacetyl)amino]-4,7-bis(dimethylamino)-1,4,4a, 3.1g (5mmol) and 2.3 g (15 mmol) of N',N'-diethylguanidine hydrochloride was reacted to obtain 2.4 g of the target compound, yield: 76.3%.
[0123] Molecular formula: C 30 h 41 N 7 o 8 Molecular weight: 627.69 Mass spectrum (m / e): 629 (M+1)
[0124] Elemental analysis: measured value: C, 57.12%; H, 6.85%; N, 15.87%;
[0125] Theoretical value: C, 57.40%; H, 6.58%; N, 15.62%;
[0126] 1 H-NMR (600MHz, CDCl 3 ): δ1.02(t, 6H), 1.44(t, 1H), 1.76(t, 1H), 2.28(s, 1H), 2.31(s, 1H), 2.34(m, 1H), 2.36(t, 1H), 2.45(d, 1H), 2.46(s, 6H), 2.60(q, 4H), 2.68(d, 1H), 2.87(s, 6H), 3.15(d, 1H), 3.58(s, 2H ), 5.16(s, 1H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com