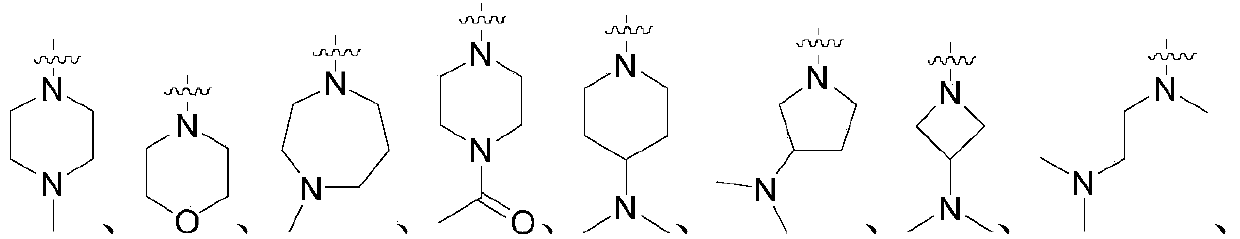
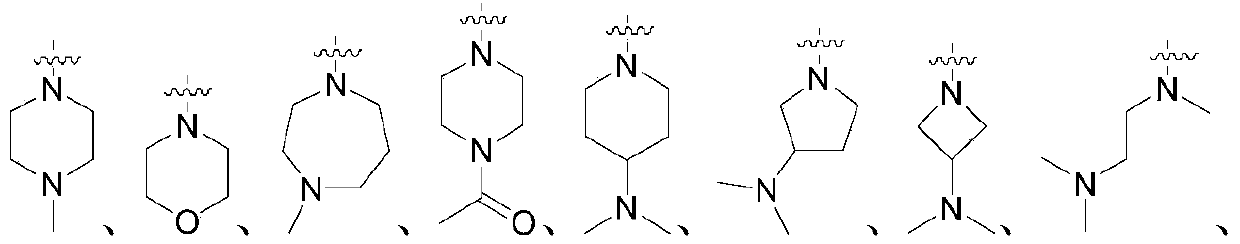
2-aminopyrimidine compounds as well as pharmaceutical compositions and applications thereof
一种化合物、氨基的技术,应用在化学医药领域,能够解决药代性质差、选择性差、不能解决药物耐药性临床压力等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0192] (E)-N-((3-((5-chloro-4-((naphthalen-2-yl)amino))pyrimidin-2-yl)amino)-4-methoxyphenyl)-4-( Dimethylamino)-2-butenamide (CCB118563)
[0193]
[0194] Step 1.2, 5-Dichloro-N-(naphthalen-2-yl)pyrimidin-4-amine (2)
[0195] 2,4,5-Trichloropyrimidine (6g, 32.7mmol), 2-naphthylamine (4.92g, 34.4mmol), sodium carbonate (6.94g, 65.4mmol) were dissolved in absolute ethanol (100mL) and stirred overnight at room temperature . Ice water (300 mL) was added with stirring, and a large amount of solid precipitated out. Filter under reduced pressure and dry under vacuum to obtain a brown solid (8.38 g, yield 88%).
[0196] 1 HNMR (400MHz, DMSO-d6): δ9.74(s,1H), 8.42(s,1H), 8.09(d, J=1.6Hz, 1H), 7.95-7.86(m,3H), 7.73(d, J=8.8Hz, 1H), 7.54-7.47 (m, 2H).
[0197] MS(ESI): m / z291[M+H] + .
[0198] Step 2.5-Chloro-N 2 -(2-Methoxy-5-nitrophenyl)-N 4 -(Naphthyl-2-yl)pyrimidine-2,4-diamine (3)
[0199] Add 2,5-dichloro-N-(naphthalene-2-yl)pyrimidin-4-amine (2) (3g, 10.3mmol), 2-methoxy-5-nitroaniline...
Embodiment 2
[0211] (E)-N-((3-((5-chloro-4-((quinolin-6-yl)amino))pyrimidin-2-yl)amino)-4-methoxyphenyl)-4- (Dimethylamino)-2-butenamide (CCB145213)
[0212]
[0213] The synthesis method is as in Example 1.
[0214] 1 HNMR (400MHz, DMSO-d6): δ 9.85 (s, 1H), 9.00 (s, 1H), 8.73 (s, 1H), 8.39 (s, 1H), 8.27 (s, 1H), 8.18 (s, 1H), 8.07-8.01 (m, 2H), 7.98 (s, 1H), 7.87 (d, J = 8.8 Hz, 2H), 7.39 (m, 1H), 7.02 (d, J = 8.8 Hz, 1H), 6.64-6.58(m,1H), 6.12(d,J=15.6Hz,1H), 3.76(s,3H), 3.00(d,J=4.8Hz,2H), 2.14(s,6H).
[0215] MS(ESI); m / z504[M+H] + .
Embodiment 3
[0217] (E)-N-((3-((5-chloro-4-((quinolin-3-yl)amino))pyrimidin-2-yl)amino)-4-methoxyphenyl)-4- (Dimethylamino)-2-butenamide (CCB145221)
[0218]
[0219] The synthesis method is as in Example 1.
[0220] 1 HNMR(400MHz,DMSO-d6): δ9.81(s,1H),9.17(s,1H),9.15(d,J=2.4Hz,1H), 8.71(d,J=2.0Hz,1H), 8.28 (s, 1H), 8.19 (s, 1H), 7.97 (d, J = 2.0 Hz, 1H), 7.91 (d, J = 8.8 Hz, 1H), 7.69 (d, J = 7.6 Hz, 1H), 7.60 (t,J=8.0Hz,1H), 7.56(dd,J=8.8Hz,2.4Hz,1H), 7.49(t,J=8.0Hz,1H), 7.01(d,J=8.8Hz,1H), 6.64-6.57(m,1H), 6.13(d,J=15.6Hz,1H), 3.76(s,3H), 3.01(d,J=5.6Hz,2H), 2.15(s,6H).
[0221] MS(ESI): m / z504[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com