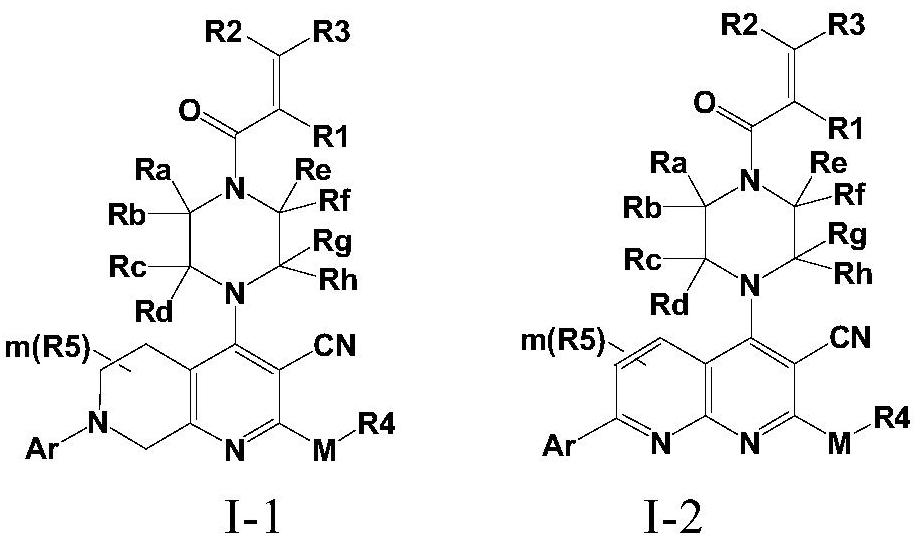
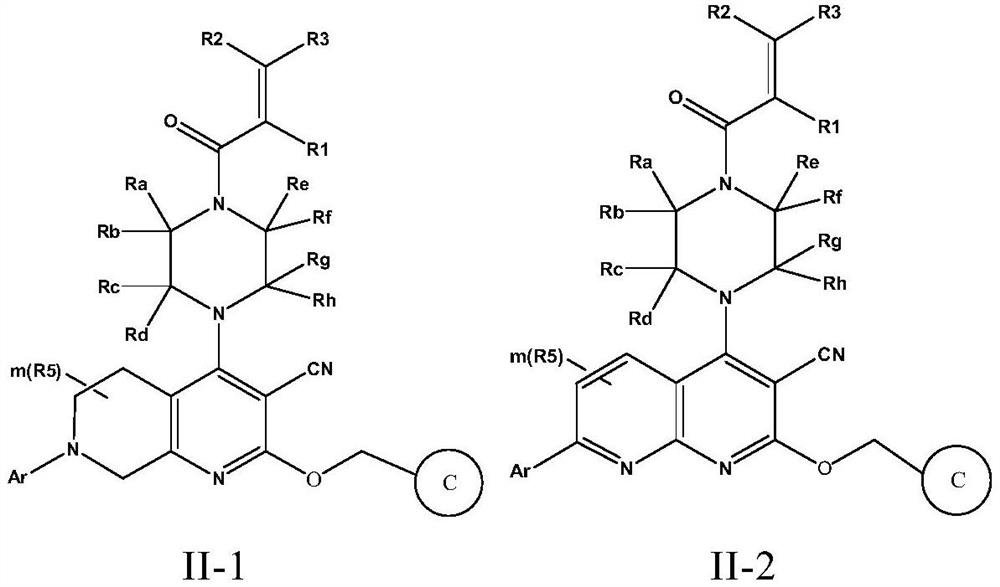
Fused cyanopyridine compound and preparation method and application thereof
A technology of cyanopyridines and compounds, applied in the field of medicinal chemistry, can solve problems such as little effect and failure to develop targeted drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0160]Example 1: (S)-4-(4-acryloylpiperazin-1-yl)-7-(5-methyl-1H-indazol-4-yl)-2-((1-methylpyrrole) Alk-2-yl)methoxy)-5,6,7,8-tetrahydro-1,7-naphthyridin-3-carbonitrile
[0161]
[0162]Step 1: Dissolve Intermediate 1 (9.5g, 31.7mmol) and N-Boc-piperazine (8.81g, 47.4mmol) in 1,4-dioxane (100mL), add diisopropylethylamine (DIEA ) (8.18g, 63.4mmol), the temperature was raised to 90 degrees and reacted overnight. The reaction solution was cooled to room temperature, concentrated under reduced pressure, and used saturated sodium bicarbonate NaHCO3The aqueous solution is slurried, filtered, and the filter cake is dried and then slurried with ethyl acetate for purification to obtain 4-(7-benzyl-3-cyano-2-hydroxy-5,6,7,8-tetrahydro-1,7-naphthalene (Pyridin-4-yl)piperazine-1-carboxylic acid tert-butyl ester (3.5 g, dark green solid). LC-MS: ESI[M+H]+=450.4;1H NMR (400MHz, DMSO_d6): δ11.53 (brs, 1H), 7.28-7.34 (m, 5H), 3.62 (s, 2H), 3.23-3.42 (m, 10H), 2.40-2.56 (m, 4H) ,1.42(s,9H).
[0163]Step 2: ...
Embodiment 2
[0168]Example 2: 4-(4-Acryloylpiperazin-1-yl)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-2-(((S)-1-methylpyrrole) Alk-2-yl)methoxy)-1,8-naphthyridine-3-carbonitrile
[0169]
[0170]The first step: 2,4-Dichloro-6-fluoro-7-(2-fluoro-6-methoxyphenyl)-1,8-naphthyridine-3-carbonitrile (670mg, 1.8mmol), 1-Cbz-piperazine (306 mg, 1.4 mmol) and DIEA (1.2 g, 9.3 mmol) were dissolved in 1.4-dioxane (20 mL) and reacted at room temperature overnight. The reaction solution was diluted with 100 mL ethyl acetate, washed twice with water, dried, and concentrated under reduced pressure to obtain 4-(2-chloro-3-cyano-6-fluoro-7-(2-fluoro-6-6-methoxy) (Phenyl)-1,8-naphthyridin-4-yl)piperazine-1-carboxylic acid benzyl ester (605 mg, red solid). LC-MS: ESI[M+H]+= 550.6.
[0171]The second step: the 4-(2-chloro-3-cyano-6-fluoro-7-(2-fluoro-6-6-methoxyphenyl)-1,8-naphthyridin-4-yl) Benzyl piperazine-1-carboxylate (605mg, 1.1mmol) was dissolved in TFA (2.5mL) and water (0.5mL), heated to 120°C and reacted overnight. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com