Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
54 results about "Cyanothymidine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Synthesis of 4′-cyanothymidine and analogs as potent inhibitors of HIV. ... 4′-Cyanothymidine inhibits HIV in A301 (Alex) ... pp 3704,1992 0040-4039/92 $3t .00 Printed in Great Britain Pergamon press plc Synthesis of 4'-Cyanotbyroidine and Analogs as Potent Inhibitors of HIV.1 Counde O-Yang, Helen Y. Wu, Elizabeth B. Praser-Smith and Keith ...
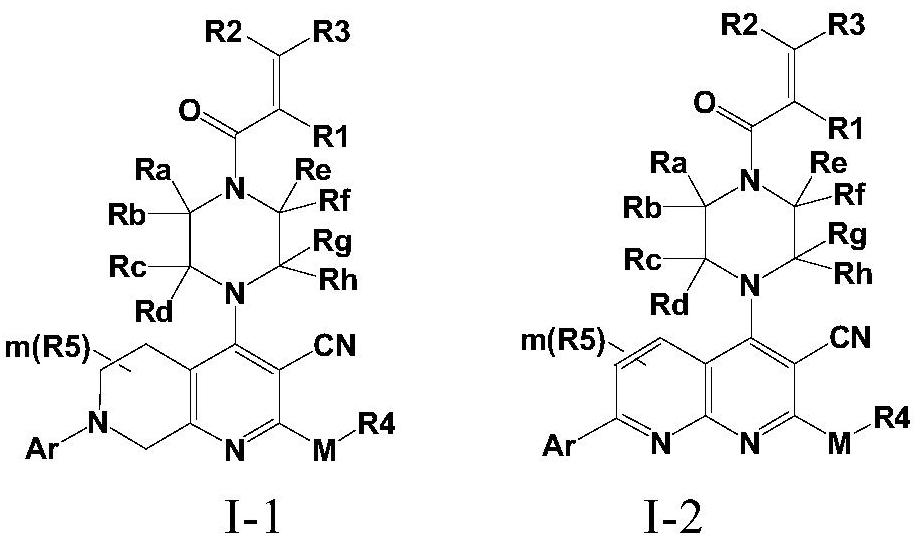
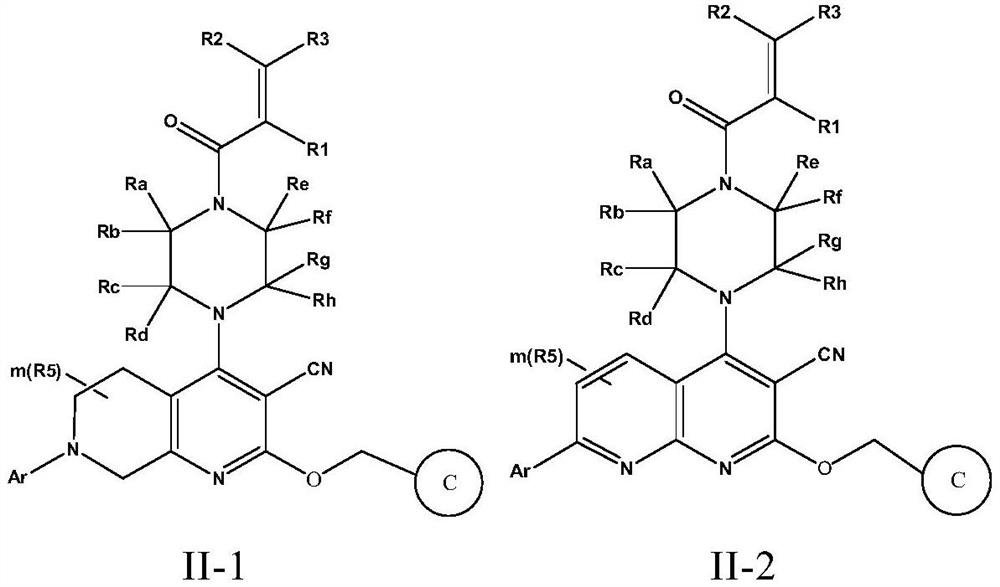
Fused cyanopyridine compound and preparation method and application thereof
ActiveCN112142735AGood selective inhibitionGood pharmacodynamicsOrganic active ingredientsOrganic chemistryCombinatorial chemistryDiastereomer
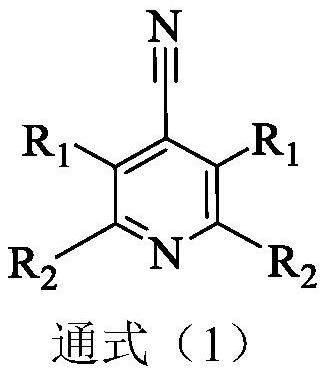
The invention discloses a fused cyanopyridine compound shown as a general formula I-1 or I-2, or a pharmaceutically acceptable salt thereof, or an enantiomer, a diastereoisomer, a tautomer, a torsional isomer, a solvate, a polymorphic substance or a prodrug thereof, and a preparation method and a pharmaceutical application of the fused cyanopyridine compound, wherein the definition of each group is shown in the specification.
Owner:RUDONG RINGENE PHARMA CO LTD +1
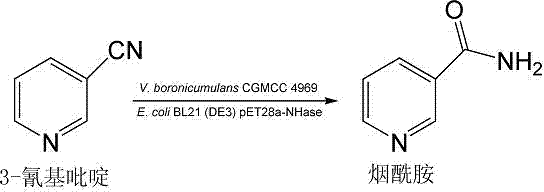
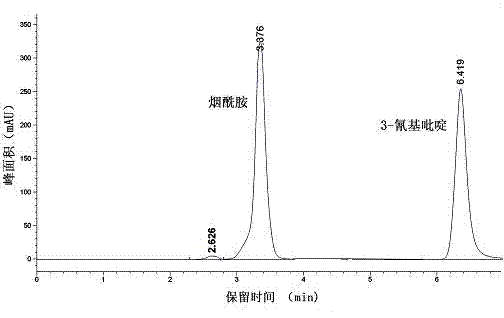
Vaporophage bacterium cgmcc4969 and its application in the biotransformation of 3-cyanopyridine to nicotinamide
The invention discloses the use of variovoraxboronicumulans CGMCC 4969 in bioconversion of 3-cyanopyridine for forming nicotinamide. The nitrile hydratase gene cluster produced by the strain consists of a DNA sequence represented by SEQ ID No.1 in a sequence table; the DNA consisting of the sequence represented by SEQ ID No.1 is recombined onto a pET28a plasmid and can be induced to express in EscherichiacoliBL21(DE3) strain; and the Escherichia coli cells containing expressed proteins and cell extracting solution can convert 3-cyanopyridine into nicotinamide.
Owner:NANJING NORMAL UNIVERSITY
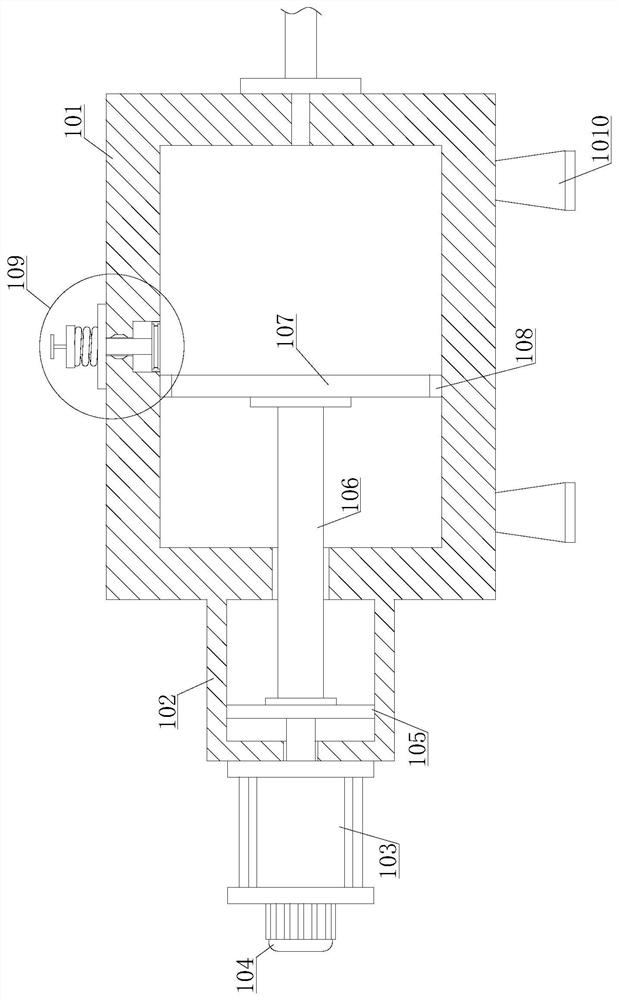
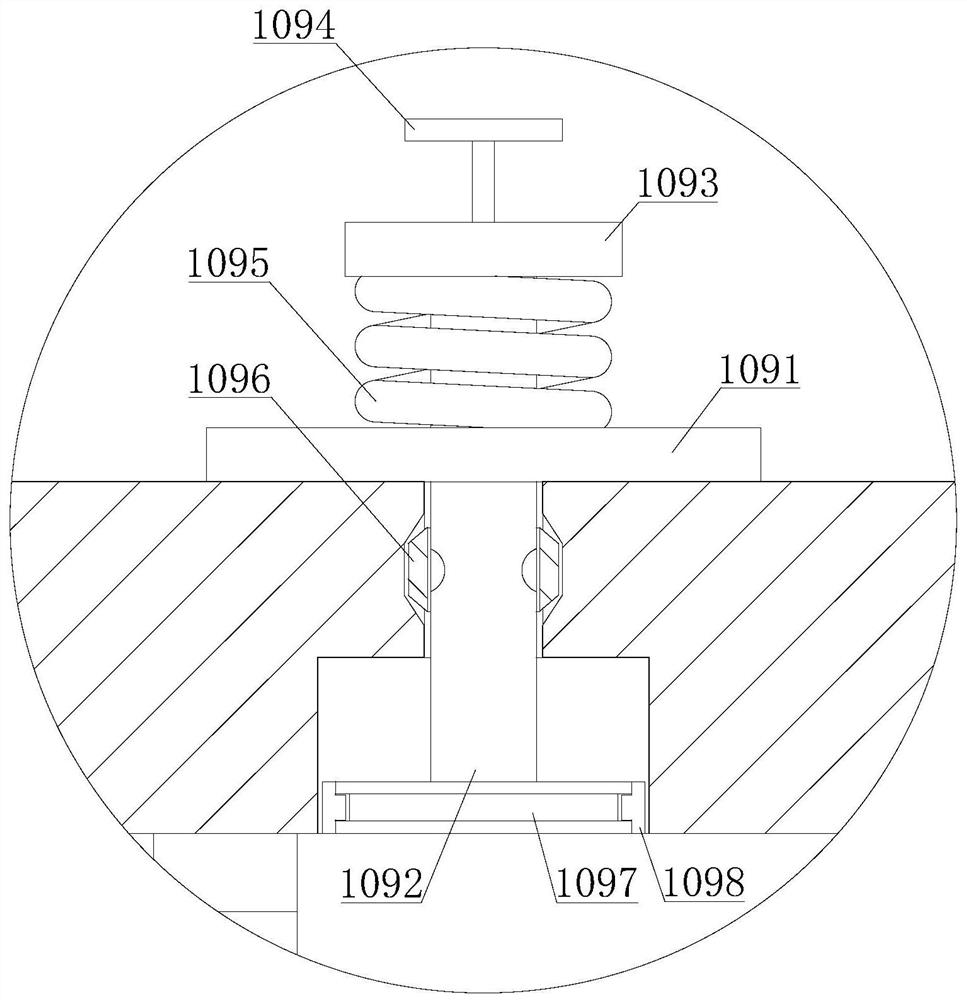
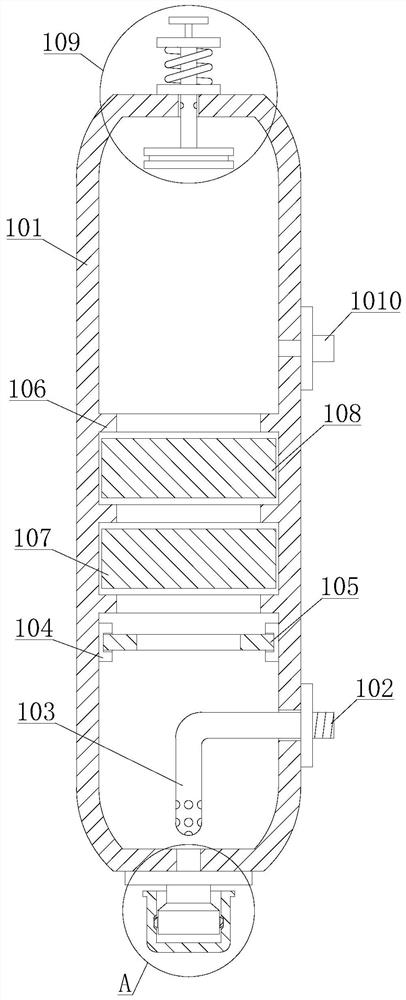
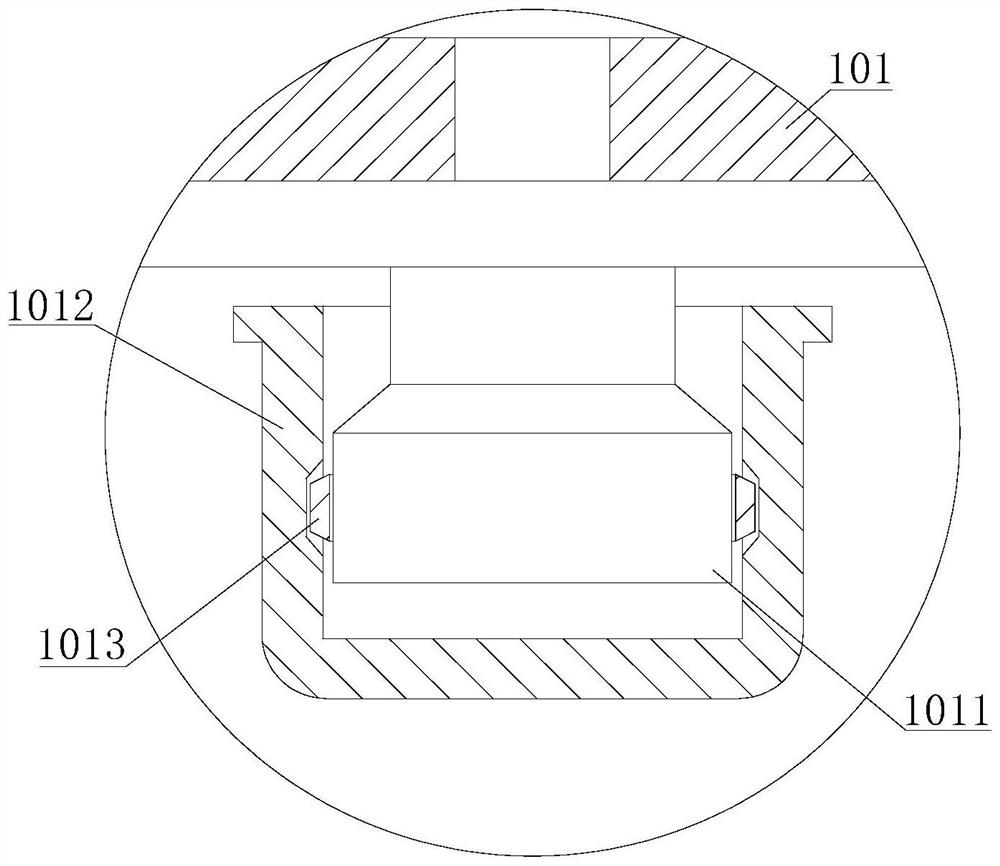
Method for purifying 3,4,5,6-tetrachloro-2-cyanopyridine by sublimation and catching, and catcher and system thereof
ActiveCN102070519ASimple processEasy to operate and controlOrganic chemistrySublimationWater chlorinationCarboxylic acid
The invention relates to preparation of a compound 3,4,5,6-tetrachloro-2-cyanopyridine, in particular to a method for catching and purifying 3,4,5,6-tetrachloro-2-cyanopyridine in a gas reaction product in a gas phase chlorination process for producing 3,4,5,6-tetrachloro-2-cyanopyridine. The method comprises the following steps: (1) primary sublimation catching: a reaction gas mixture containing 3,4,5,6-tetrachloro-2-cyanopyridine from a reaction bed enters a first catcher, an inert gas is introduced, the gases are thoroughly mixed and quenched to 135-165 DEG C, and sublimated flocculent crystals fall to a material outlet at the bottom of the first catcher by gravity and are discharged; and (2) secondary sublimation catching: the gas which is not sublimated in the primary sublimation catching enters a second catcher, an inert gas is introduced, gases are thoroughly mixed and quenched to 55-110 DEG C, sublimated flocculent crystals fall to a material outlet at the bottom of the second catcher by gravity and are discharged, and the gas which is not sublimated enters a bubbling absorber. In the invention, the solid product can be further hydrolyzed to obtain high-purity 3,4,5,6-tetrachloropyridine-2-carboxylic acid; and the invention has the characteristic of simple technique, and is easy to operate and control.
Owner:SHANDONG SHENGBANG LUNAN PESTICIDE
Novel cyanopyridine derivatives useful in the treatment of cancer and other disorders
This invention relates to novel diaryl ureas of Formula (I), pharmaceutical compositions containing such compounds and the use of those compounds or compositions for treating hyperproliferative and angiogenesis disorders, as a sole agent or in combination with cytotoxic therapies.
Owner:BAYER HEALTHCARE LLC
Synthesis and application of antineoplastic 2-amino-3-cyano pyridine
InactiveCN102924372ARapid responseImprove responseOrganic active ingredientsOrganic chemistrySynthesis methodsStomach cancer
The invention provides a novel antineoplastic drug shown in a molecular formula (I). The synthesis method of the drug is simple and convenient, and the drug can be quickly and efficiently synthesized through microwave-assisted one-pot four-component reaction. The synthesis method comprises the following specific steps: dissolving 2 mmol of p-hydroxybenzaldehyde, 2 mmol of 4-fluoroacetophenone or 4-chloropropiophenone and 2 mmol of malononitrile in 1,4-dioxane; then adding 4 mmol of ammonium acetate; performing microwave (300W)-assisted heating to 120 DEG C, and reacting for 20 minutes; and adding ice water into the reaction mixture to precipitate a product, and then recrystallizing to obtain the product, namely 2-amino-3-cyano pyridine. The compound obtained by the invention is a novel antineoplastic drug shown in the molecular formula (I), and has the activity of obviously inhibiting the proliferation of liver cancer, stomach cancer, mammary cancer, pancreatic cancer, brain cancer, lung cancer and ovarian cancer cells.
Owner:CHINA PHARM UNIV +1
Continuous evaporative crystallization device and method for 4-cyanopyridine
ActiveCN112691403ALower crystallization temperatureSimplified crystallization stepsOrganic chemistryEvaporator accessoriesPhysical chemistryOrganosolv
The invention discloses a continuous evaporative crystallization device and method for 4-cyanopyridine. The device comprises a pressurizing assembly, a connecting pipe and an evaporation reaction kettle, and is characterized in that the pressure in the evaporation reaction kettle is increased by the pressurizing assembly so as to the crystallization temperature of the 4-cyanopyridine and the boiling point temperature of other organic solvents, after pressurization is completed, a heating resistor is used for heating and crystallizing part of the solution to ensure that only water and other organic solvents are vaporized and the 4-cyanopyridine is heated and crystallized in the evaporation and crystallization process of the 4-cyanopyridine, and meanwhile, water vapor, the other organic solvents and part of the 4-cyanopyridine are absorbed and liquefied again by a condensation reflux box, and drop on the right side of the evaporation reaction kettle, and the heating resistor on the right side carries out evaporative crystallization operation under normal pressure again, so that the 4-cyanopyridine is separated out after being crystallized and is attached to the inner wall of the right side of the evaporation reaction kettle, the crystallization operation steps of the 4-cyanopyridine are simplified, and the crystallization amount of the 4-cyanopyridine separated out is effectively increased.
Owner:ANHUI COSTAR BIOCHEM CO LTD
Ion palladium activation liquid and preparation method thereof, and plastic surface chemical plating method
ActiveCN105463417ASimple processLiquid/solution decomposition chemical coatingChemical platingCopper chloride
The present invention provides an ion palladium activation liquid and a preparation method thereof, and a plastic surface chemical plating method. The ion palladium activation liquid comprises a soluble palladium salt, copper chloride, a chloride complexing agent, 1,10-phenanthroline and 4-cyanopyridine, and the pH value of the ion palladium activation liquid is 6-12. According to the present invention, the service life of the activation liquid is long, the plating time of the material activated with the activation liquid is short, no overflow plating or leakage plating phenomenon is generated, and the adhesion is strong.
Owner:BYD CO LTD
Preparation method of substituted pyridine
ActiveCN111320572AIncrease mass transfer rateEnhanced mass transferOrganic chemistryProcess engineeringNitrogen gas
The invention discloses a preparation method of substituted pyridine. The method is implemented in a jet loop reactor based on a Venturi effect. First nitrogen displacement is performed, then, acrylicacid and 4-cyanopyridine raw materials are sequentially added through a material feeding opening; an outer circulating pump is started at 70 to 110 DEG C for high-speed spraying and mixing; a hydrogen chloride raw material is slowly added into the jet loop reactor through a gas raw material feeding hole; spraying and mixing are performed through a spraying type mixer, and full reaction among materials is realized; and the like. According to the method, a novel jet loop reactor based on the Venturi effect is adopted; a traditional stirred tank reactor is replaced; according to the reactor, themass transfer among various reaction species in the reactor is greatly promoted, so that the reaction can be dynamically controlled, and when reaction raw materials are sprayed and pushed forwards under the action of the pump, strong suction force can be generated to automatically suck gas-phase reaction materials in the reaction kettle, so that an excellent mass transfer effect is obtained in violent turbulent flow.
Owner:杭州瑞思新材料有限公司
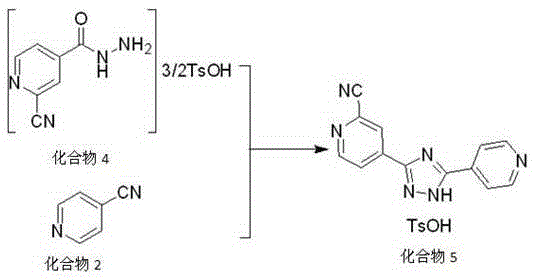
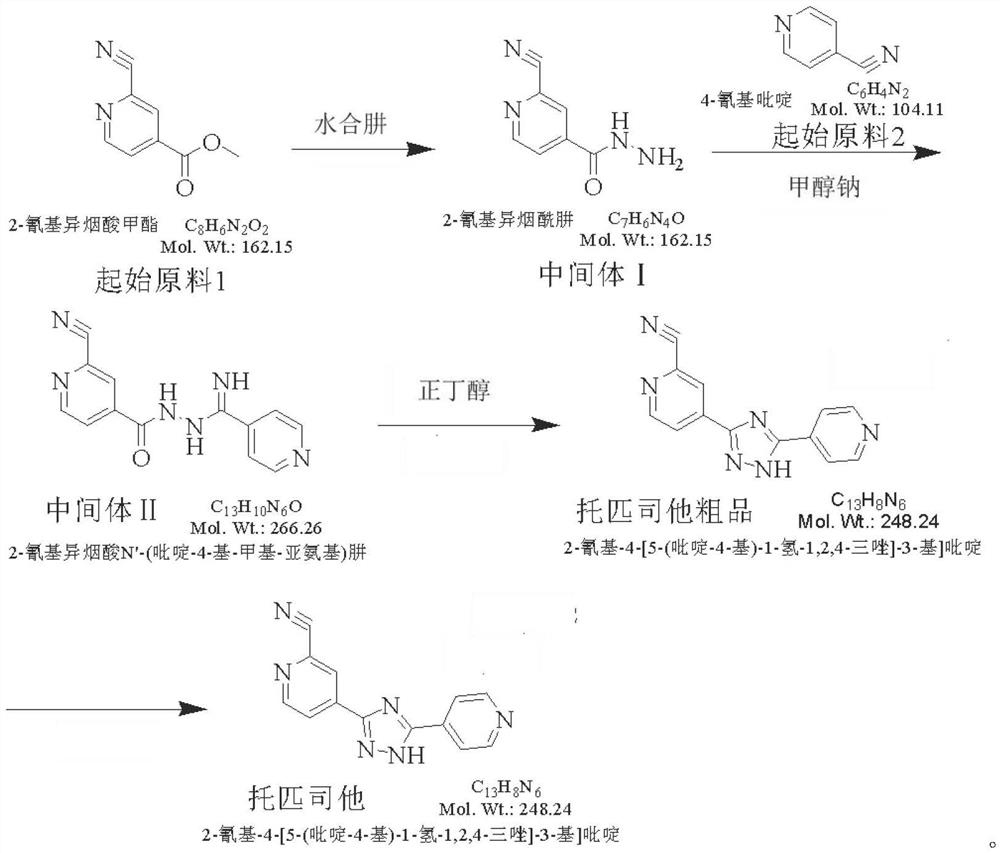
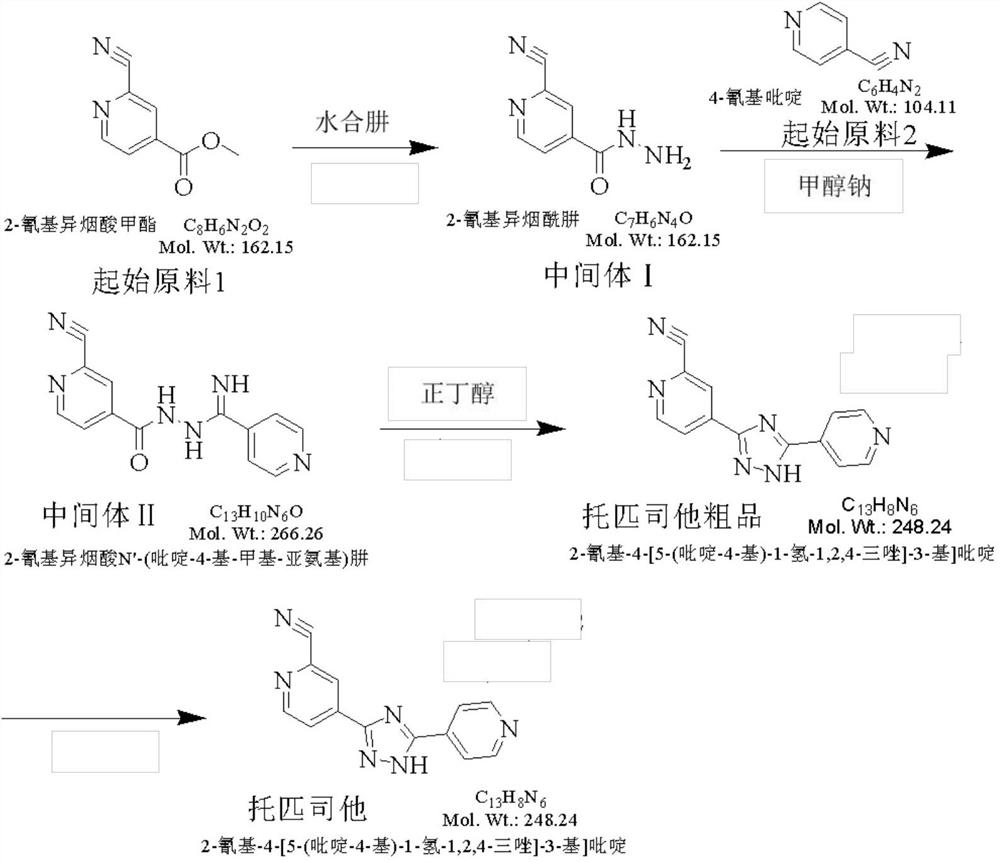
Preparation method of topiroxostat
InactiveCN105399732AReasonable choice of methodSimple process routeOrganic chemistryActivated carbonOxide
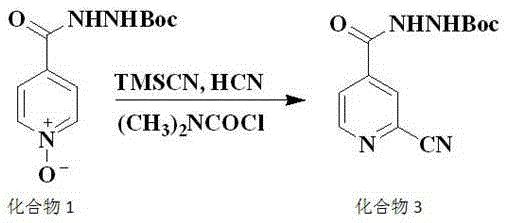
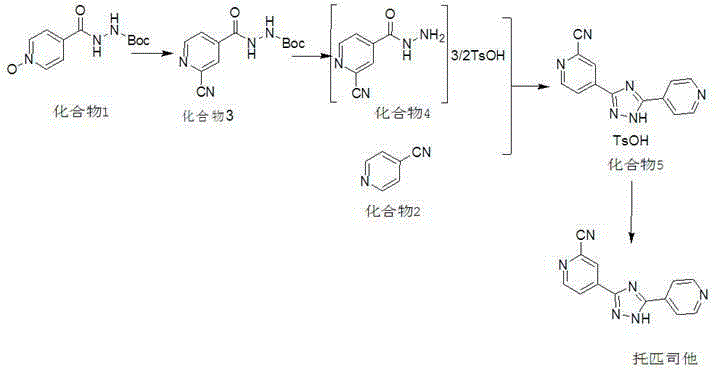
The invention discloses a preparation method of topiroxostat, and belongs to the technical field of medicine synthesis and organic compound synthesis and preparation. N2-Boc-isonicotinic acid hydrazide-(compound 1)oxide and 4-cyanopyridine(compound 2) serve as the initial raw materials, and a compound 1 is subjected to cyanation, protect base removal and salifying to form a compound 4; the compound 4 and a compound 2 are subjected to a ring closing reaction and salifying to form a compound 5, and the compound 5 is subjected to salifying and refining, impurity removing achieved through activated carbon, alkali regulating and drying to form a high-purity product. By means of the method, the defects of the prior art are overcome, and the method has the advantages that raw materials are stable and easy to obtain, reaction conditions are mild, the technological process is simple, convenient and easy to control and the yield is high; the method is suitable for industrial production.
Owner:CP PHARMA QINGDAO CO LTD
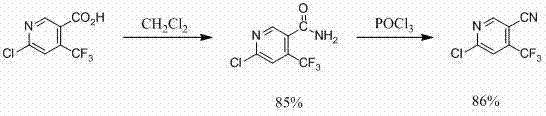
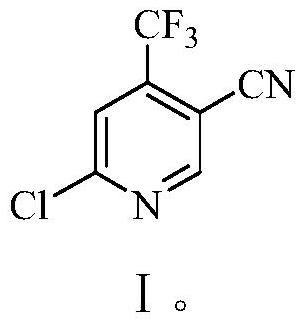
Method for preparing 6-chlorine-4-trifluoromethyl-3-cyanopyridine
The invention discloses a method for preparing 6-chlorine-4-trifluoromethyl-3-cyanopyridine. The method comprises steps of synthesizing 2-chlorine-6-methoxy-4-trifluoromethyl-3-cyanopyridine, synthesizing 6-methoxy-4-trifluoromethyl-3-cyanopyridine, synthesizing 6-hydroxy-4-trifluoromethyl-3-cyanopyridine, and synthesizing 6-chlorine-4-trifluoromethyl-3-cyanopyridine. The method has high productivity and short reaction steps, is simple to operate, is efficient and is easy to carry out.
Owner:贵州威顿晶磷电子材料股份有限公司
Synthesis method of topiroxostat
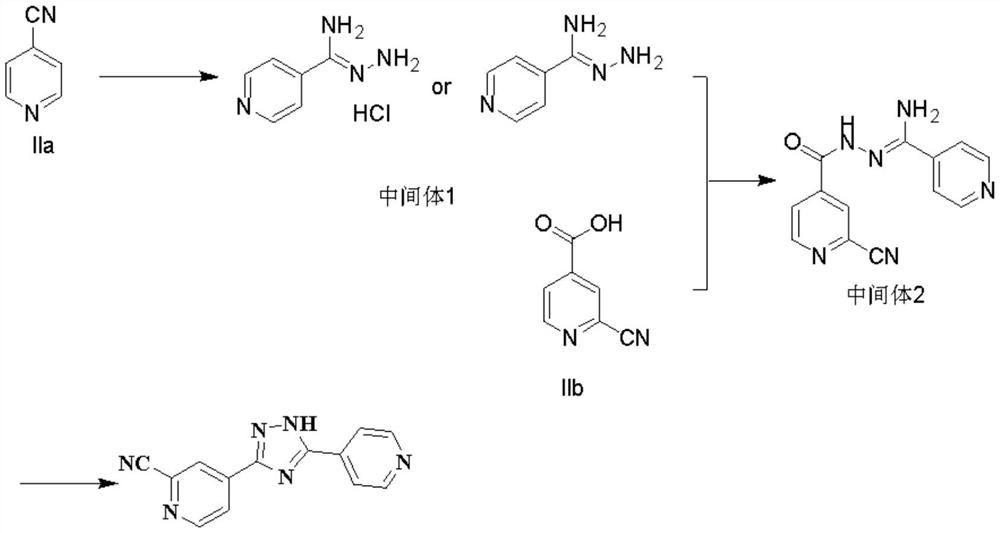
The invention provides a synthesis method of topiroxostat, and relates to the technical field of medicine synthesis. The synthesis method of topiroxostat comprises the steps of carrying out heat-preservation stirring reaction on a raw material 4-cyanopyridine and 80% hydrazine hydrate in the presence of an alcohol solvent and an alkaline reagent, and carrying out post-treatment to obtain an intermediate 1, or carrying out post-treatment under the condition of hydrochloric acid to obtain a hydrochloride form of the intermediate 1; reacting the intermediate 1 or the hydrochloride form of the intermediate 1 with 2-cyano-4-picolinic acid in the presence of a solvent and a condensing agent, and performing post-treatment to obtain an intermediate 2; and heating and refluxing the intermediate 2 for 2-4 hours under the condition of acetic acid, cooling to room temperature, filtering, and carrying out forced air drying to obtain the topiroxostat (crystal form I). The synthesis method has the advantages of low production cost, high yield, high purity and less three wastes, and is suitable for industrial production of topiroxostat and intermediates thereof.
Owner:南京安一合医药科技有限公司
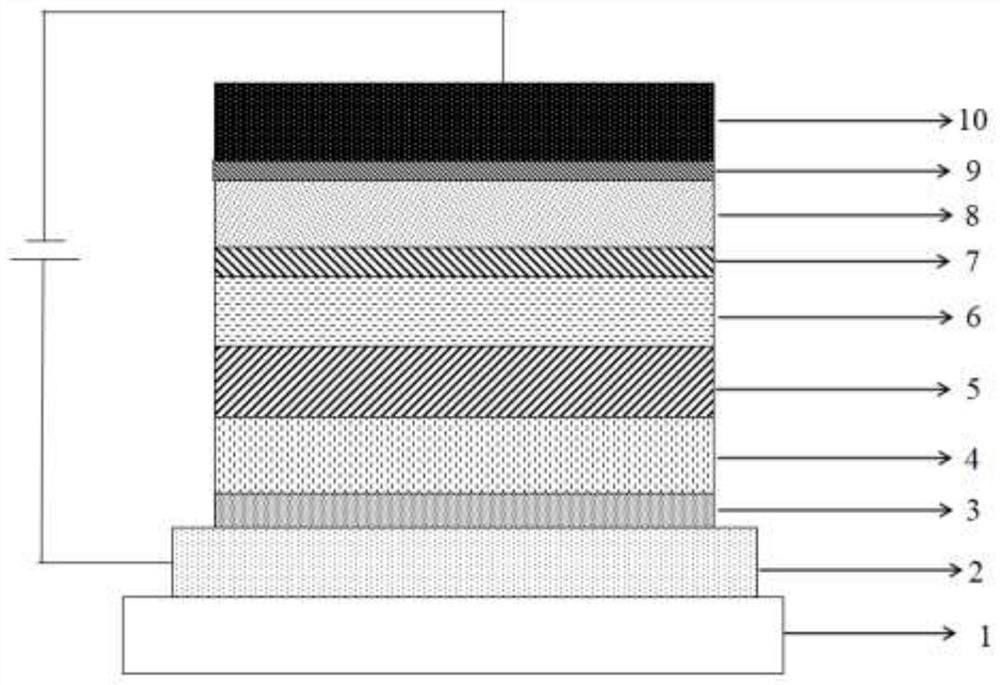
Organic compound taking 4-cyanopyridine as core and organic electroluminescent device comprising same
PendingCN114105868AImprove stabilityExtend your lifeOrganic chemistrySolid-state devicesSimple Organic CompoundsOrganic electroluminescence
The invention relates to an organic compound taking 4-cyanopyridine as a core and an organic light-emitting device comprising the same, belongs to the technical field of semiconductors, and provides a compound with a structure shown in a general formula (1), and also discloses an organic light-emitting device comprising the compound. The compound provided by the invention has relatively high thermal stability, can generate a TADF effect when being used as a luminescent layer material of an OLED luminescent device, has relatively high PLQY and relatively small spectral change, and can effectively improve the photoelectric property of the OLED device and prolong the service life of the OLED device.
Owner:JIANGSU SUNERA TECH CO LTD
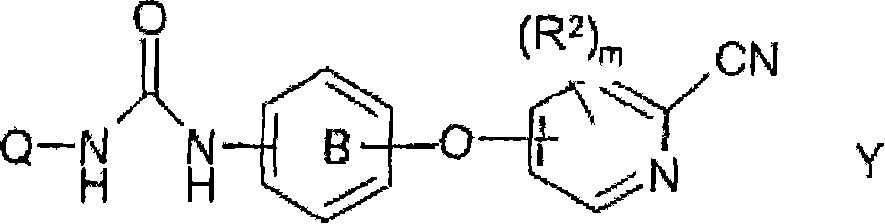
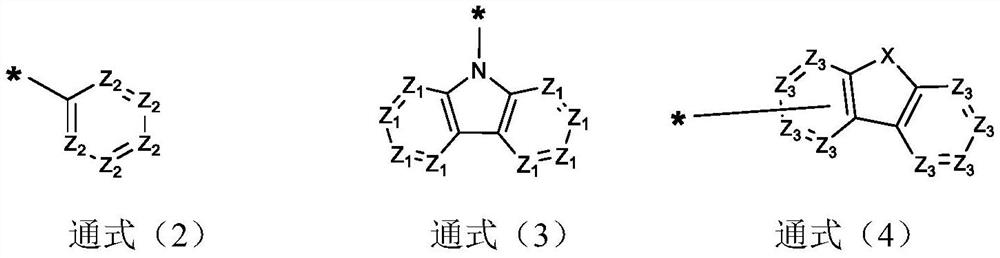
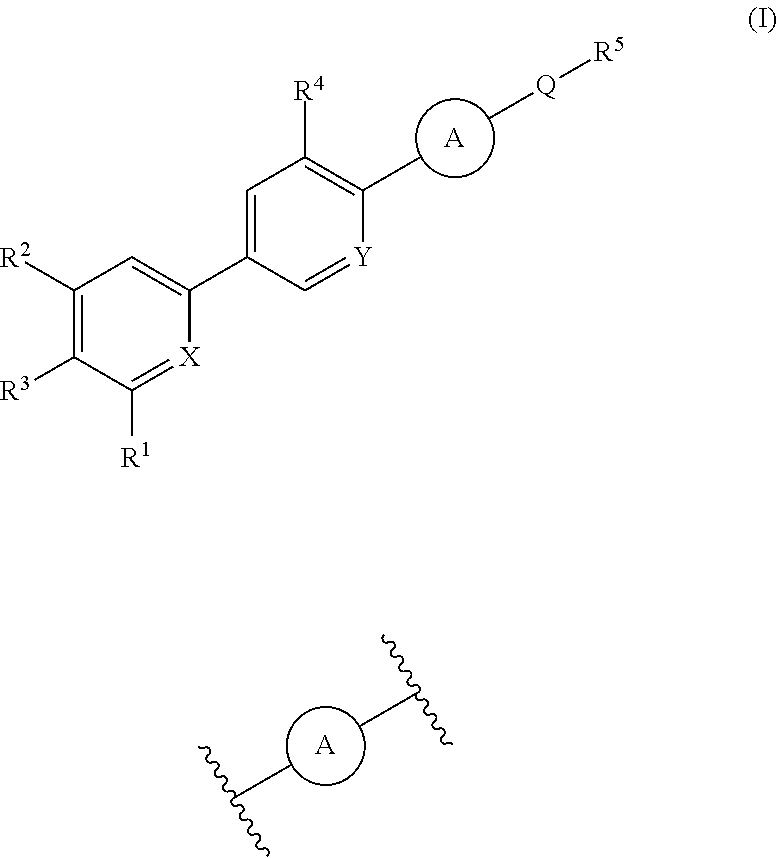
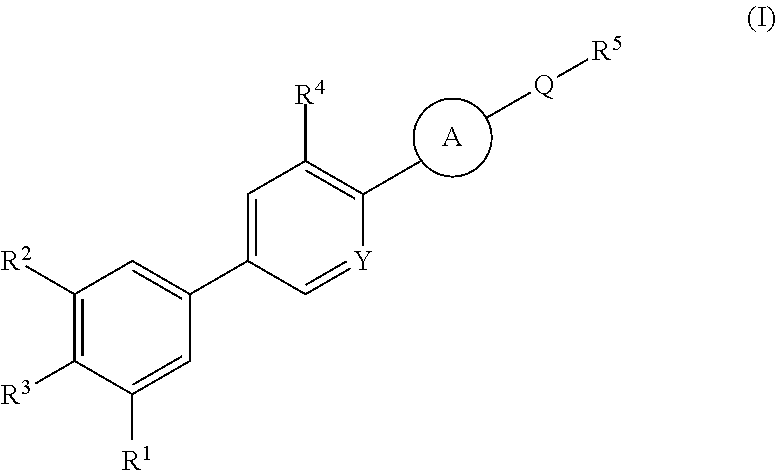
Cyanopyridine Derivatives as Liver X Receptor Beta Agonists, Compositions, and Their Use
In its many embodiments, the present invention provides substituted cyanopyridine containing compounds of the Formula (I): and acceptable salts thereof, wherein R1, R2, R3, R4, R5, X, Y, Q, and the moiety are as defined herein. The novel compounds of the invention, and pharmaceutically acceptable compositions comprising a compound thereof, are useful as Liver X-β receptor (LXRβ) agonists, and may be useful for treating or preventing pathologies related thereto. Such pathologies include, but are not limited to, inflammatory diseases and diseases characterized by defects in cholesterol and lipid metabolism, such as Alzheimer's disease.
Owner:MERCK SHARP & DOHME LLC
Preparation method of bionic immobilized 3-cyanopyridine nitrile hydratase
InactiveCN104894092AAdvantages of catalytic hydrolysis performanceImprove repeated use stabilityOn/in organic carrierHydrolysisNitrile hydratase
The invention discloses an indium oxide nanosphere coating brevibacterium flavum nitrile hydratase, a preparation method and application. The multilayered structure of cells is simulated by referring to the existence form of the hydratase in a living body, and the indium oxide nanosphere is prepared through bionic design of components, functions and processes and used for embedding of the brevibacterium flavum nitrile hydratase. The characteristics that the immobilized nitrile hydratase is high in stability, high in activity, easy to recycle and the like are used for catalyzing 3-cyanopyridine to be transformed into nicotinamide in an efficient hydrolysis mode. The preparation method of the bionic immobilized hydratase can provide reference significance for preparation of a microcyst immobilized carrier, and provide a novel research idea for biotransformation of the 3-cyanopyridine.
Owner:ANHUI COSTAR BIOCHEM CO LTD
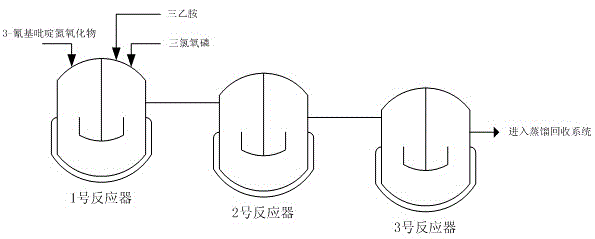
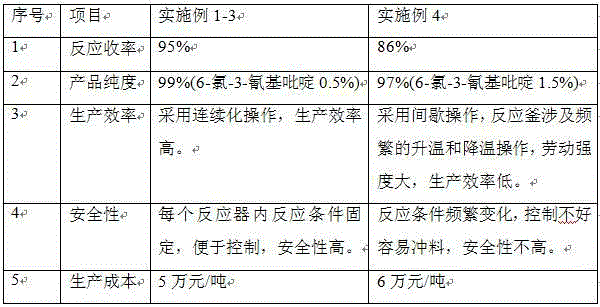
Method for producing 2-chloro-3-cyanopyridine through continuous reaction
The invention belongs to the field of organic synthesis, and particularly relates to a method for producing 2-chloro-3-cyanopyridine. The method is implemented by taking nicotinonitrile-1-oxide, phosphorus oxychloride and triethylamine as raw materials through the steps that the three raw materials are simultaneously continuously dropwise added into a first reactor, and the reaction temperature is controlled at minus 10-40 DEG C; and a reaction liquid in the first reactor continuously enters subsequent reactors connected in series to react at a reaction temperature of 80-100 DEG C, 1-2 hours later, the reaction liquid is distilled for recovering phosphorus oxychloride, and the phosphorus oxychloride is subjected to hydraulic analysis, so that 2-chloro-3-cyanopyridine is obtained. The method is implemented by adopting a continuous reaction thought, and reaction conditions in the related reactors are smooth, so that the implementation of automation control is facilitated, therefore, the method has the advantages of safe process, high production efficiency, stable product quality, and the like.
Owner:JIANGSU ZHONGZHENG BIOCHEM CO LTD
Method for separating 4-cyanopyridine by cooling solvent crystallization
ActiveCN110746352AHigh purityEasy to operateOrganic chemistryCrystallization separationCentrifugationPhysical chemistry
The invention provides a method for separating 4-cyanopyridine through solvent cooling crystallization. The method comprises the following steps: S1, preparing a 4-cyanopyridine saturated solution; S2, cooling the saturated solution to obtain a mixed slurry; S3, carrying out centrifugal filtration on the mixed slurry, washing with pure water, and carrying out centrifugal filtration and drying to obtain highly-pure 4-cyanopyridine; S4, performing reduced pressure distillation on the obtained filtrate to obtain a solute, heating and decolorizing the solute, and repeating step S1 and step S2 to obtain highly-pure 4-cyanopyridine; and S5, collecting the highly-pure 4-cyanopyridine obtained in step S3 and step S4 to obtain the finished 4-cyanopyridine product. The purity of the 4-cyanopyridineseparated through cooling crystallization is high, and reaches up to 99.54%; the primary filtrate and the washing filtrate are distilled and then purified, so the yield of 4-cyanopyridine is increased, and can reach up to 97.2%; and a separation and purification device for 4-cyanopyridine can complete a series of steps of crystallization, centrifugation, washing, drying and the like in separationand purification of 4-cyanopyridine.
Owner:ANHUI RUIBANG BIOLOGICAL SCI & TECH CO LTD
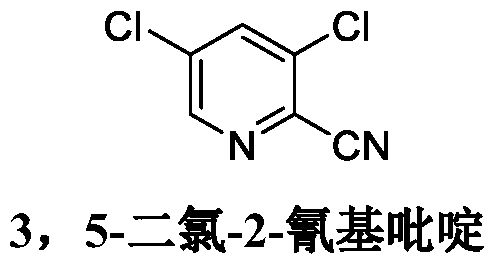
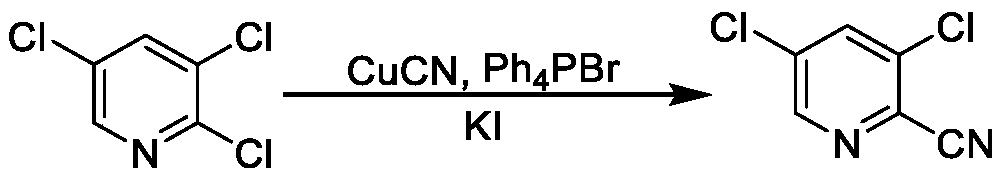
Synthesis method of 3, 5-dichloro-2-cyanopyridine
The invention provides a synthesis method of 3, 5-dichloro-2-cyanopyridine. The method includes the steps of: 1) in the presence of a solvent, mixing 2, 3, 5-trichloropyridine with a fluoride for reaction to obtain 3, 5-dichloro-2-fluoropyridine; and 2) mixing the 3, 5-dichloro-2-fluoropyridine prepared in step 1) with a catalyst and a cyanide salt for reaction to obtain 3, 5-dichloro-2-cyanopyridine. The method provided by the invention uses sodium cyanide, potassium cyanide or other cheap and easily available cyanide salts as the cyaniding reagent with a dosage far lower than that of the existing method, and can acquire the target product with high yield and high content, thereby greatly reducing the production cost, also greatly reducing the production of wastewater in the three wastes,and being an efficient and environment-friendly production method of 3, 5-dichloro-2-cyanopyridine.
Owner:CHONGQING MEDICAL & PHARMA COLLEGE
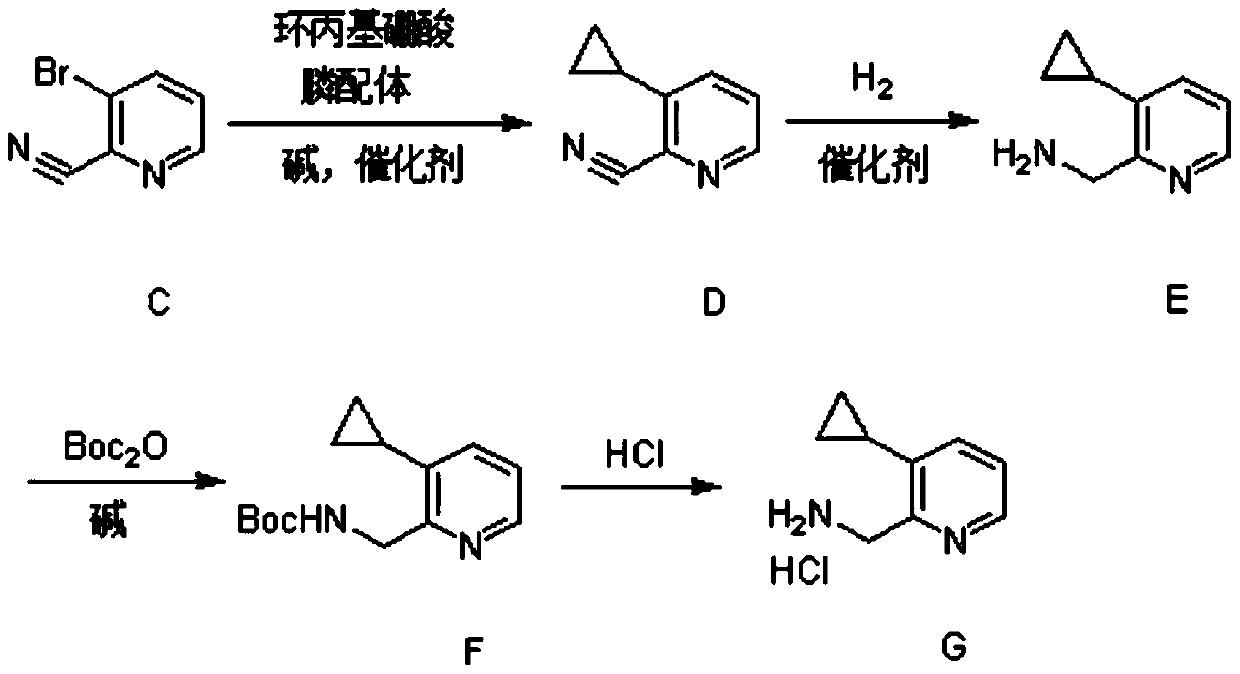
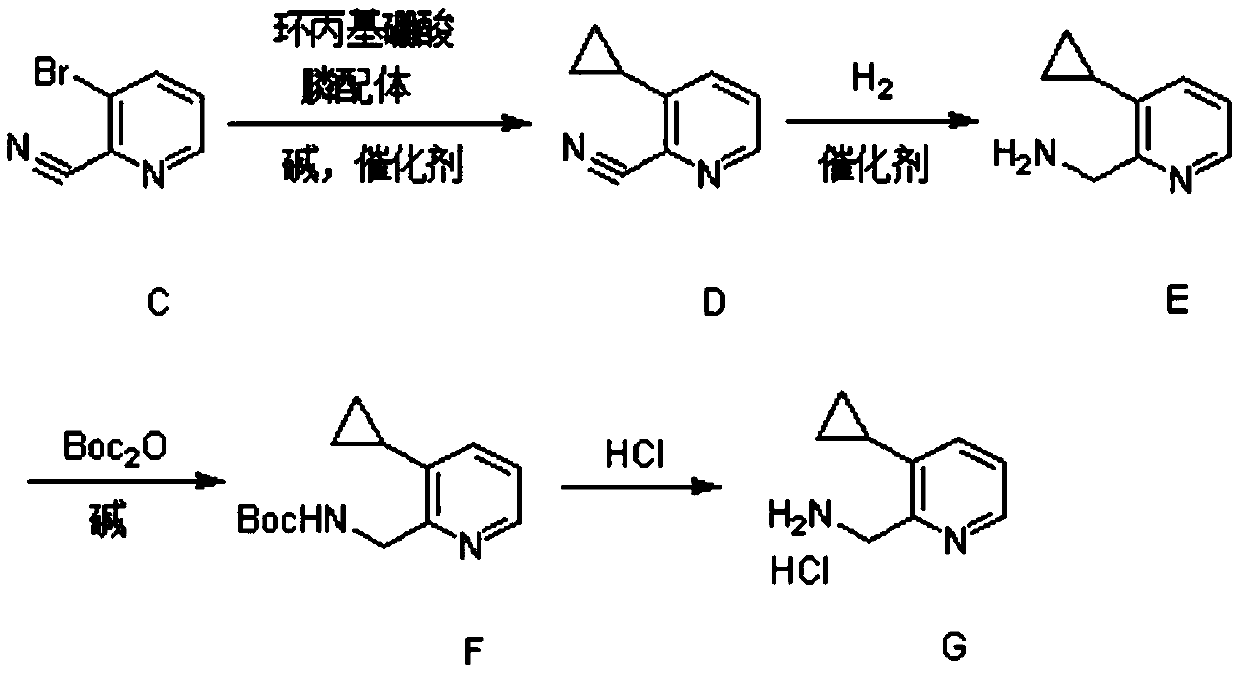
Synthesis method of (3-cyclopropylpyridin-2-yl) methylamine hydrochloride
InactiveCN111285798AReasonable designExperiment operation is simpleOrganic chemistryPtru catalystPhosphine
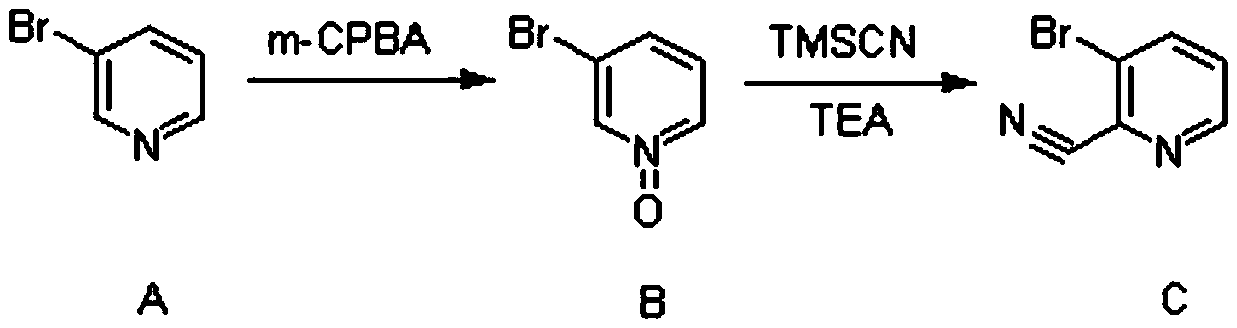
The invention provides a synthesis method of (3-cyclopropylpyridin-2-yl) methylamine hydrochloride, and belongs to the technical field of synthesis of organic chemical intermediates. The preparation method comprises the following steps: reacting 3-bromo-2-cyanopyridine with cyclopropylboronic acid, a phosphine ligand, an alkali and a catalyst under the protection of nitrogen, then reacting with hydrogen under the action of the catalyst, reacting with di-tert-butyl dicarbonate and alkali, and finally reacting with an organic solvent solution of hydrogen chloride to obtain a target product (3-cyclopropylpyridin-2-yl) methylamine hydrochloride. The 3-bromo-2-cyanopyridine is prepared by taking 3-bromopyridine as a raw material and carrying out nitrogen oxidation and cyanation. The method is reasonable in process design, simple in experimental operation and easy to control.
Owner:阿里生物新材料(常州)有限公司
Rectification and separation device and method for 2-cyanopyridine base material
InactiveCN112717455AEfficient separationAvoid pollutionLiquid degasificationDispersed particle separationThermodynamicsPhysical chemistry
The invention discloses a rectification separation device and method for a 2-cyanopyridine backing material. The method comprises the following steps: pouring a prepared backing material into a heating stirring kettle, driving a stirring shaft and stirring blades to stir by a stirring motor in the heating stirring kettle, and heating in cooperation with a heating resistor, so that ammonia water in the backing material is decomposed into ammonia gas in an accelerated manner; ammonia gas is mixed with other gases and guided into the rectification separation tower through the heat preservation connecting pipe, firstly, the sulfuric acid solution at the bottom of the rectification shell is bubbled through the gas outlet guide pipe, so that the sulfuric acid solution absorbs a large amount of ammonia gas, and meanwhile, a small part of ammonia gas and other gases are upwards absorbed through the filter screen plate, the coarse filter filler and the fine filter filler; and finally, the residual gas is conveyed to the liquefaction assembly through the heat preservation connecting pipe, liquefied through the liquefaction assembly and then guided into the heating stirring kettle again through the conveying pipe, the heating stirring kettle continues to conduct heating and stirring to conduct the next rectification separation cycle, ammonia water in the prepared base material is effectively separated, and the situation that the ammonia water is decomposed into ammonia gas and pollutes the environment is avoided.
Owner:ANHUI COSTAR BIOCHEM CO LTD
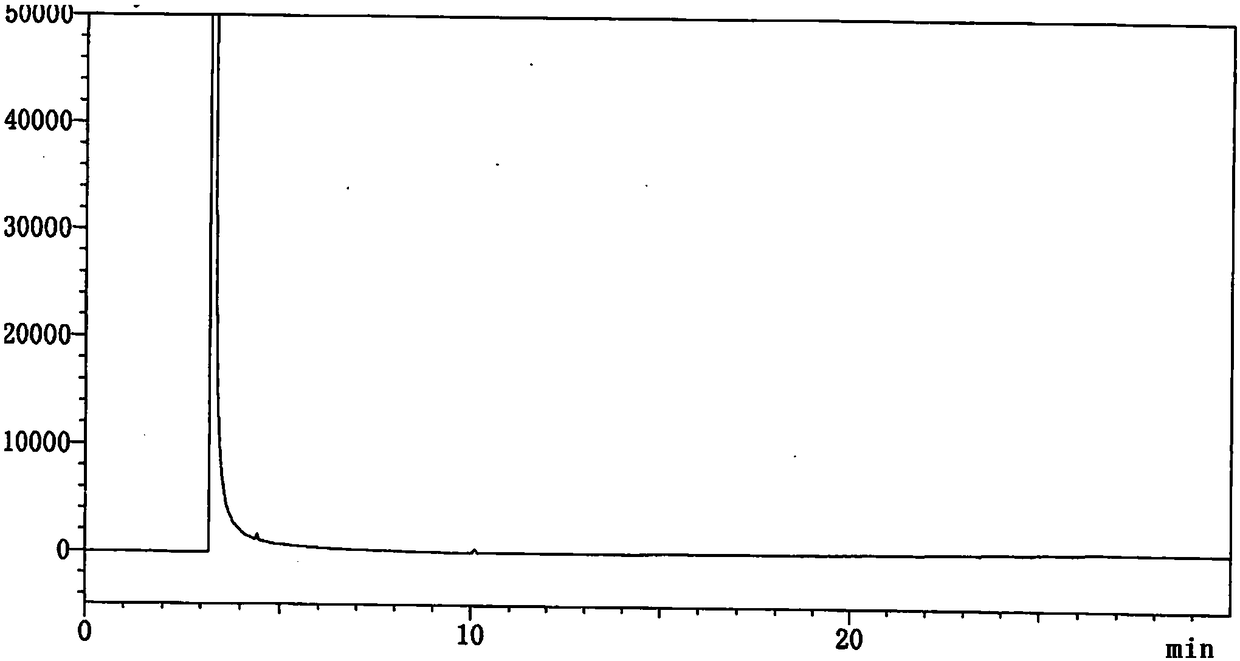
Method for determining content of 4-cyanopyridine and impurities of 4-cyanopyridine in isoniazide starting material
InactiveCN108226323AEfficient separationDo not interfere with detectionComponent separationProduct gasVaporization
The invention belongs to the field of analytical chemistry, and particularly relates to a method for determining the content of 4-cyanopyridine and impurities of 4-cyanopyridine in an isoniazide starting material. The measuring method is a gas chromatographic method, and comprises the following specific steps: vaporizing a sample in a vaporization chamber, and introducing the vaporized sample intoa chromatographic column through flowing gas; separating the 4-cyanopyridine SM1 and the impurities of 4-cyanopyridine by means of programmed heating; calculating the content of the impurities by using a principal component self-contrasted method with a correction factor. At present, no reports of simultaneous separation and determination of a plurality of impurities in the SM1 exist. The methodfor measuring the content of the plurality of impurities in the SM1 provided by the invention is simple and feasible, and has high sensitivity and high specificity, so that established quality controlitems of SM1 relevant substances can improve the quality controllability and safety of a product when being applied to control of relevant substances of the product.
Owner:CHONGQING HUABANGSHENGKAI PHARM
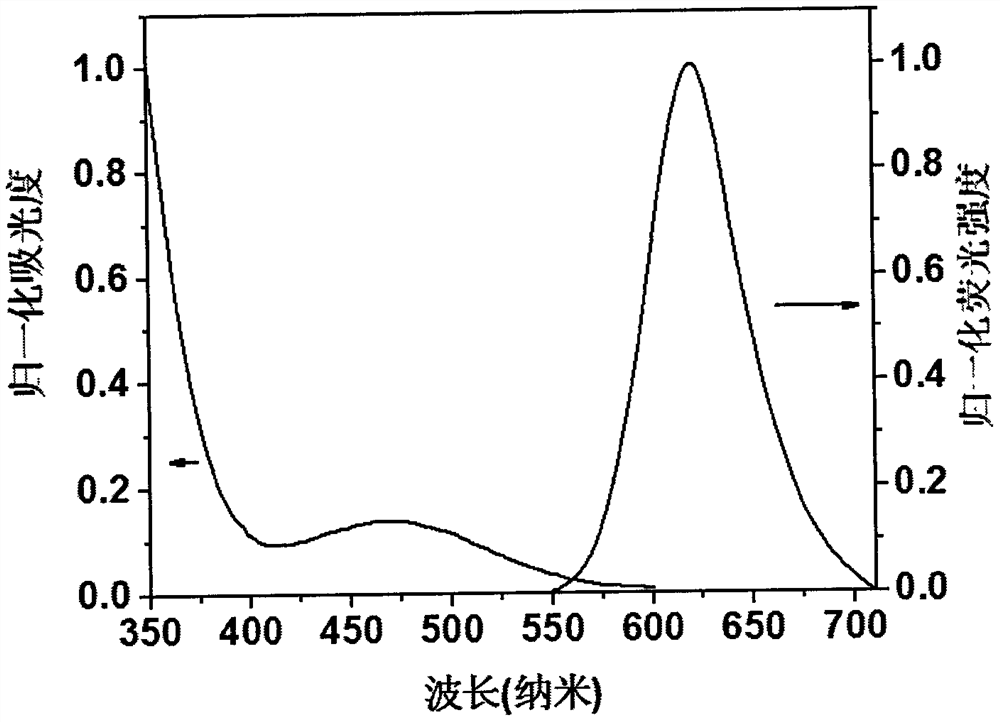
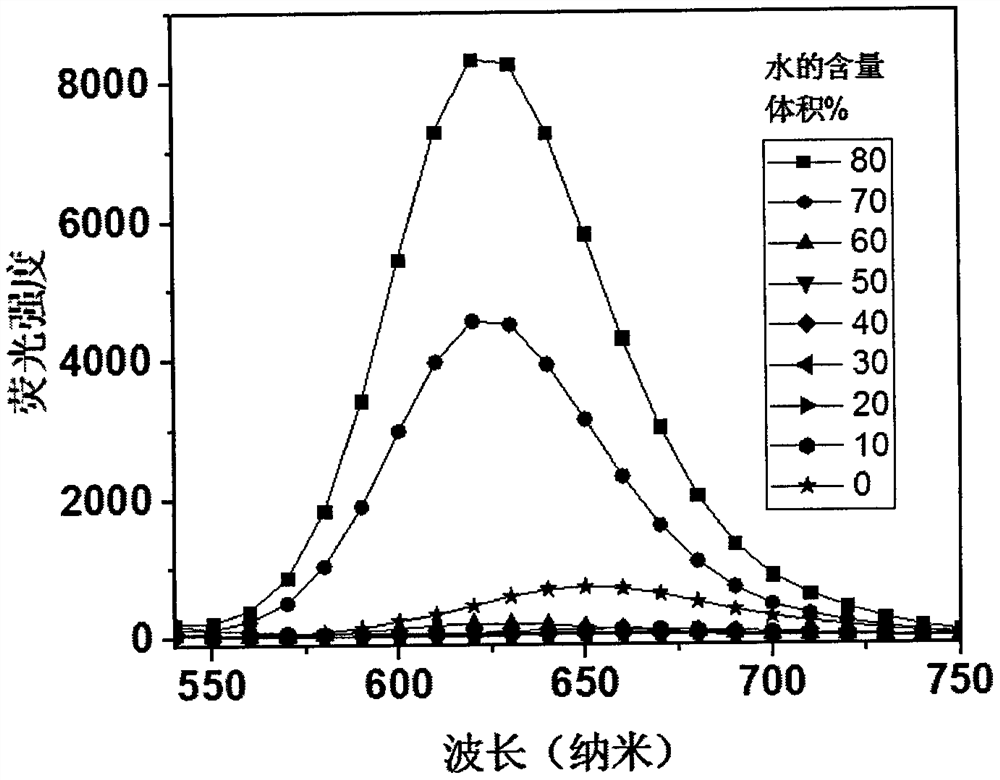
Preparation method and application of a solid-state acid-base stimuli-responsive near-infrared fluorescent compound
ActiveCN110483381BHas aggregation-induced luminescent propertiesHighly aggregated state luminescenceOrganic chemistryLuminescent compositionsChemical structureSolid acid
The invention provides a solid-state acid-base stimulus-responsive near-infrared fluorescent compound 1, the molecular chemical formula of which is shown in formula (I): the invention discloses a solid-state acid-base stimulus-response The preparation method and application of the near-infrared fluorescent compound. Among them, triphenylamine and cyanopyridine are common fluorescent compound units, but the unique chemical structure makes this compound have the advantages of aggregation-induced luminescence characteristics and high solid-state luminescence, and can respond to acid-base stimuli. Under the light, it can be observed that the fluorescence changes from the deep red-near-infrared region at 652nm to the fluorescence emission at 789nm near-infrared I region, with a shift of up to 137nm. In daylight, it can be seen that it changes from red to blue-black. Therefore, the present invention provides a solid-state acid-base stimulus-responsive near-infrared fluorescent material, which has broad application prospects in the fields of stimulus-responsive switch molecular devices, sensing, and anti-counterfeiting.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
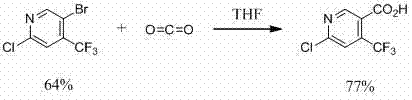
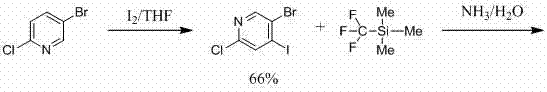
Preparation method of 6-chloro-4-trifluoromethyl-3-cyanopyridine
ActiveCN113912535AHigh yieldHigh purityOrganic chemistryBulk chemical productionMethacrylateAminopropionitrile
The invention provides a preparation method of 6-chloro-4-trifluoromethyl-3-cyanopyridine, which comprises the following steps: by taking 2-chloro-3-trifluoromethyl acrylate (II) and 2-chloro-3-aminopropionitrile (III) as raw materials, carrying out addition reaction to obtain 2,4-dichloro-3-trifluoromethyl-4-cyano-5-amino valerate (IV), carrying out molecular lactamization reaction to obtain 3,5-dichloro-4-trifluoromethyl-5-cyano piperidine-2-one (V), then removing hydrogen chloride to obtain 6-hydroxy-4-trifluoromethyl-3-cyanopyridine (VI), and then carrying out chlorination reaction on VI and a chlorination reagent to obtain 6-chloro-4-trifluoromethyl-3-cyanopyridine (I). The raw materials used in the invention are cheap and easily available; the preparation and operation method is simple, small in wastewater amount, safe, environment-friendly and low in cost; the method has the advantages of high reaction selectivity, few byproducts and high target product yield and purity, and is suitable for green industrial production.
Owner:XINFA PHARMA
Method for purifying 3,4,5,6-tetrachloro-2-cyanopyridine by sublimation and catching, and catcher and system thereof
ActiveCN102070519BSimple processEasy to operate and controlOrganic chemistrySublimationWater chlorinationCarboxylic acid
Owner:SHANDONG SHENGBANG LUNAN PESTICIDE
An integrated recombinant Mycobacterium smegmatis producing niacin and its construction method
ActiveCN108384739BSimple production conditionsNo pollution in the processBacteriaMicroorganism based processesChemical synthesisNucleotide
The invention discloses an integrated recombinant mycobacterium smegmatis producing nicotinic acid and a construction method thereof. The invention requires the protection of a recombinant mycobacterium smegmatis producing nicotinic acid, the recombinant mycobacterium smegmatis producing nicotinic acid is characterized in that the recombinant mycobacterium smegmatis contains recombinant integrative plasmid, which contains nudC gene, and the nucleotide sequence is shown as SEQ ID NO:1. Nicotinic acid can be directly obtained by one step through the engineering strain prepared by the invention without adding 3-cyanopyridine, therefore various shortcomings in the existing chemical synthesis process of nicotinic acid can be avoided, and also the method has the characteristics of simple production condition, no pollution, simplicity and feasibility, easy enlargement and low cost, is suitable for large-scale industrial production and application, and has great popularization and applicationvalue.
Owner:上海晶诺生物科技有限公司
Preparation method of medicine for treating chronic hyperuricemia
InactiveCN112645931AHigh yieldQuality improvementOrganic chemistrySkeletal disorderSolubilityTopiroxostat
The invention discloses a preparation method of a medicine for treating chronic hyperuricemia. The method comprises the following steps: using methyl 2-cyanoisonicotinate and 4-cyanopyridine as initial raw materials, and carrying out hydrazinolysis reaction, condensation reaction, cyclization reaction and purification to obtain topiroxostat. On one hand, by using a hydrochloric acid alcohol solution, the energy consumption of the process is reduced, and the problem of poor safety is improved; and on the other hand, topiroxostat hydrochloride is refined and concentrated in alcohol water and then subjected to a salt dissolving process, so that the solubility of the topiroxostat in an alkaline solution is increased, the problem that chloride exceeds the standard is preliminarily solved, the salt dissolving process is more thorough, and the reaction proceeding degree is increased. Finally, in the purification process, the amount of chlorides in the product is further reduced by a method of hot melting the crude product in alcohol water, and the product quality is ensured; and the purity of the product is improved by selecting a purification solvent.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Preparation method of 2-bromoindolizine derivative
The invention discloses a preparation method of a 2-bromoindolizine derivative. The preparation method comprises the following steps: putting bromoacetophenone-4-cyanopyridine quaternary ammonium salt, bromoethynylbenzene, tetramethyl-1-piperidinyloxy, potassium carbonate and a solvent into a reactor, and reacting at 90 DEG C; and after the reaction is finished, pouring the obtained mixture into water, and carrying out extraction, drying and column chromatography separation to obtain the pure 2-bromoindazine derivative. The used raw materials can be directly purchased, are easy to obtain and are low in price. The used solvent has high boiling point, is non-volatilize and is safe to operate and use. The synthesis method is wide in substrate application range, and synthesis of the target product with various structures is easy to realize. The target product can be obtained by the only one-step reaction in the technological process, and the reaction is completed within 2 h. The method avoids resource and energy loss caused by separation of intermediates and tedious operation caused by the step-by-step reaction, reduces time consumption, and greatly improves the synthesis efficiency.
Owner:HUAIYIN TEACHERS COLLEGE
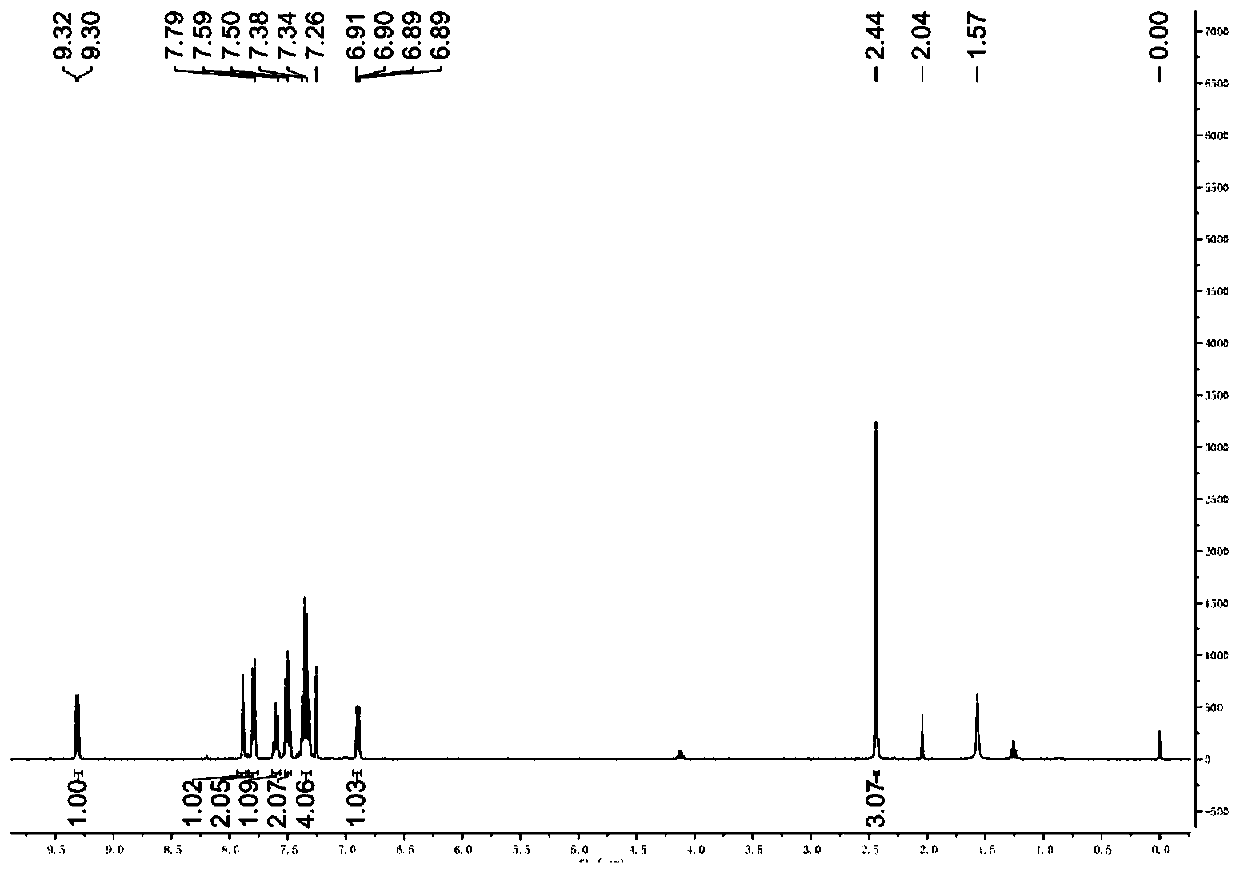
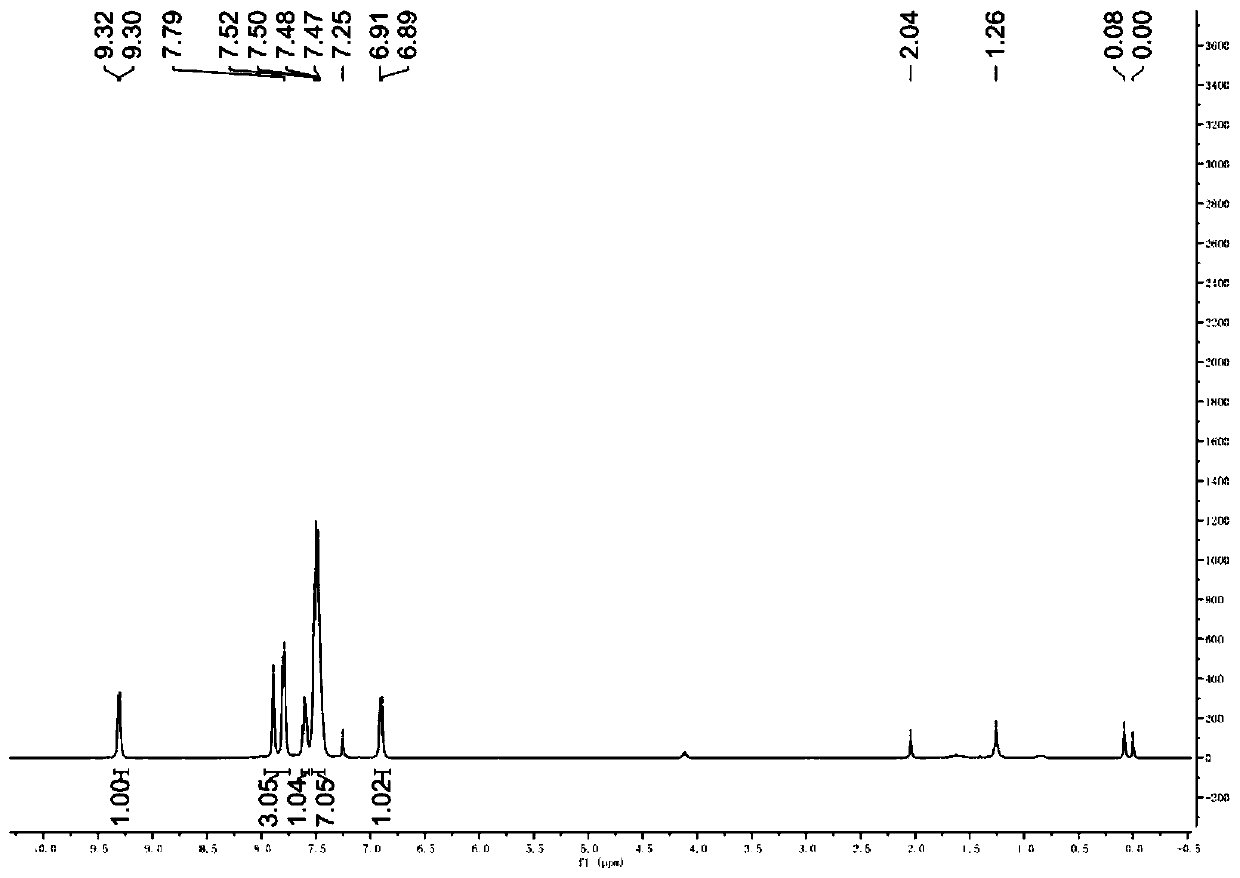
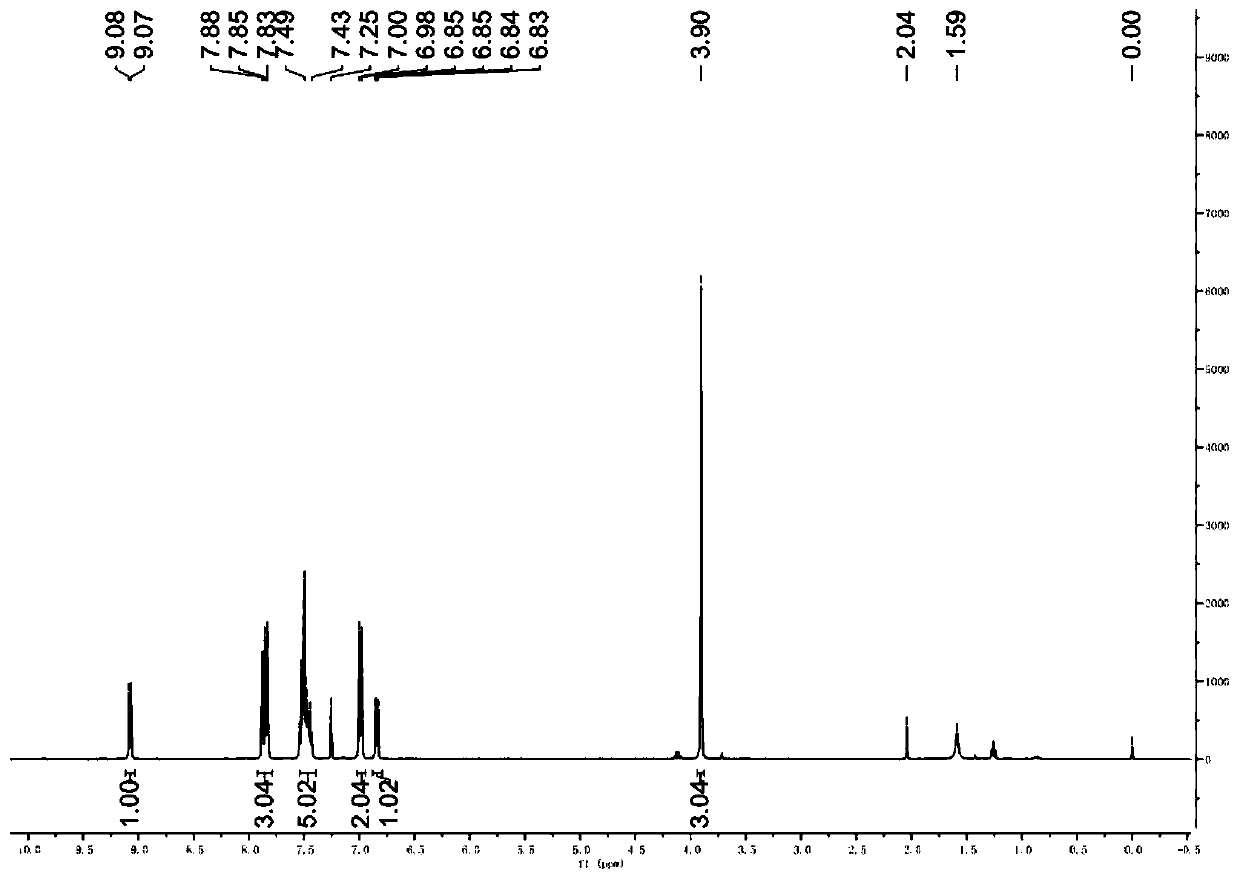
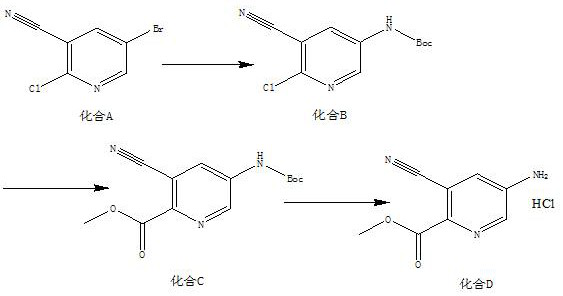
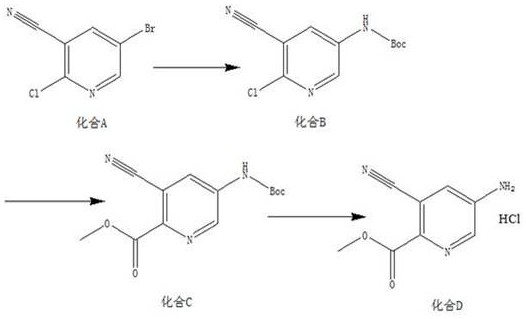
A kind of synthetic method of 5-amino-3-cyanopyridine carboxylic acid methyl ester hydrochloride
The invention belongs to pharmaceutical intermediates, in particular to a method for synthesizing methyl 5-amino-3-cyanopyridine carboxylate hydrochloride. The present invention provides a kind of compound A as basic raw material, synthesized 5-amino-3-cyanopyridine carboxylate hydrochloride through three-step reaction, is 5-amino-3-cyanopyridine carboxylate hydrochloride The synthetic method of salt provides synthetic route; The synthetic method of 5-amino-3-cyanopicolinate methyl ester hydrochloride of the present invention is that route is brief, reasonable in design, and simple to operate, easy to control; The obtained product of the present invention obtains The rate is higher.
Owner:阿里生物新材料(常州)有限公司
A strain of Escherichia coli and its application in biocatalytic production of low by-product nicotinamide
ActiveCN112195117BAdaptableEnhanced inhibitory effectBacteriaMicroorganism based processesBiotechnologyMicrobiology
The invention discloses an Escherichia coli strain and its application in biocatalytic production of low-by-product nicotinamide. The Escherichia coli M910001 described in the invention has a preservation number of CGMCC NO.20430. The Escherichia coli X described in the present invention after the transformation of Escherichia coli M910001 can normally carry out cell metabolism and growth and reproduction in 0-5% 3-cyanopyridine solution, 0-50% nicotinamide solution and their mixed solution, and adapt to the environment Strong ability; after 50 generations of continuous passage, the plasmid retention rate is above 91%, and after 100 generations, the plasmid retention rate is above 80%, which has better plasmid stability.
Owner:ANHUI RUIBANG BIOLOGICAL SCI & TECH CO LTD
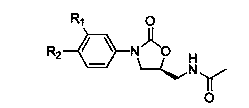
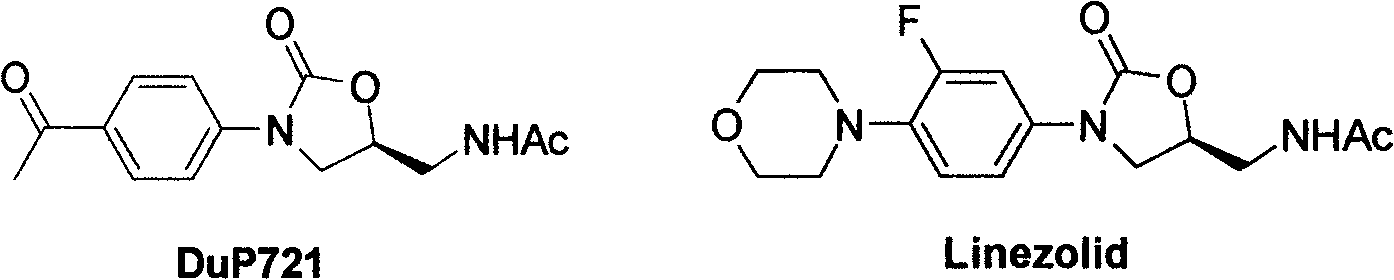
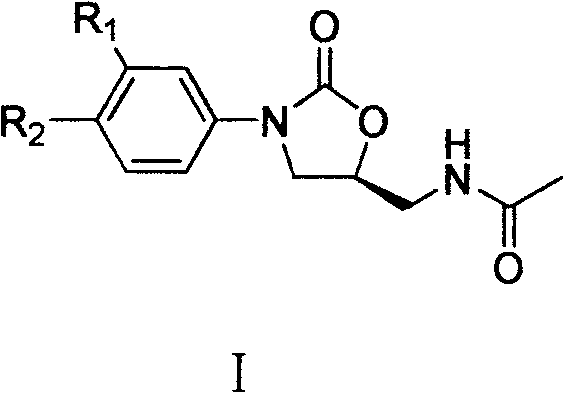
Cyanopyridyl-replaced oxazolidinone compound
InactiveCN101619061BAntibacterial agentsOrganic active ingredientsAntibacterial activityMethyl palmoxirate
The invention relates a cyanopyridyl-replaced oxazolidinone compound with a general formula (I); wherein, R1 is hydrogen, fluorine, chlorine or trifluoromethyl and R is shown in the right structural formula. The invention also provides a preparation method of the compound shown in general formula (I) and medicinal salts of the compound and an application thereof for curing microbial infections, in particular to bacterial infection diseases. Preliminary antibacterial activity tests in vitro show that the compound of the invention has better antibacterial activity compared with linezolid and obvious antibacterial activity to drug-resistant bacteria.
Owner:SHENYANG PHARMA UNIVERSITY
A mutant Mycobacterium smegmatis secreting niacin and its construction method
ActiveCN108424871BAvoid disadvantagesSimple production conditionsBacteriaMicroorganism based processesBiotechnologyChemical synthesis
The present invention provides a method for constructing a mutant Mycobacterium smegmatis secreting niacin, comprising steps: 1). Constructing plasmid pMHS-NrtR ms ; 2). Construct plasmid pHAGE-NrtR ms ; 3). Preparation of recombinant TM4 phage; 4). Construction of nrtR ms Mycobacterium smegmatis mc 2 100. The beneficial effects of the present invention are: by constructing nrtR ms The gene deletion strain Mycobacterium smegmatis enables the strain to directly prepare nicotinic acid without relying on the addition of 3-cyanopyridine and enzyme catalysis, not only avoiding various shortcomings in the existing chemical synthesis process of nicotinic acid, but also the production conditions Simple, pollution-free, easy to operate, easy to enlarge, low cost, suitable for large-scale industrial production and application, and has great value for popularization and application.
Owner:FOSHAN UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com