Preparation method of substituted pyridine
A technology of pyridine and cyanopyridine, applied in the field of preparation of substituted pyridine, can solve the problems of high price of 2-vinylpyridine, hinder industrial application, unsatisfactory recycling effect, etc., and achieve the effect of saving energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
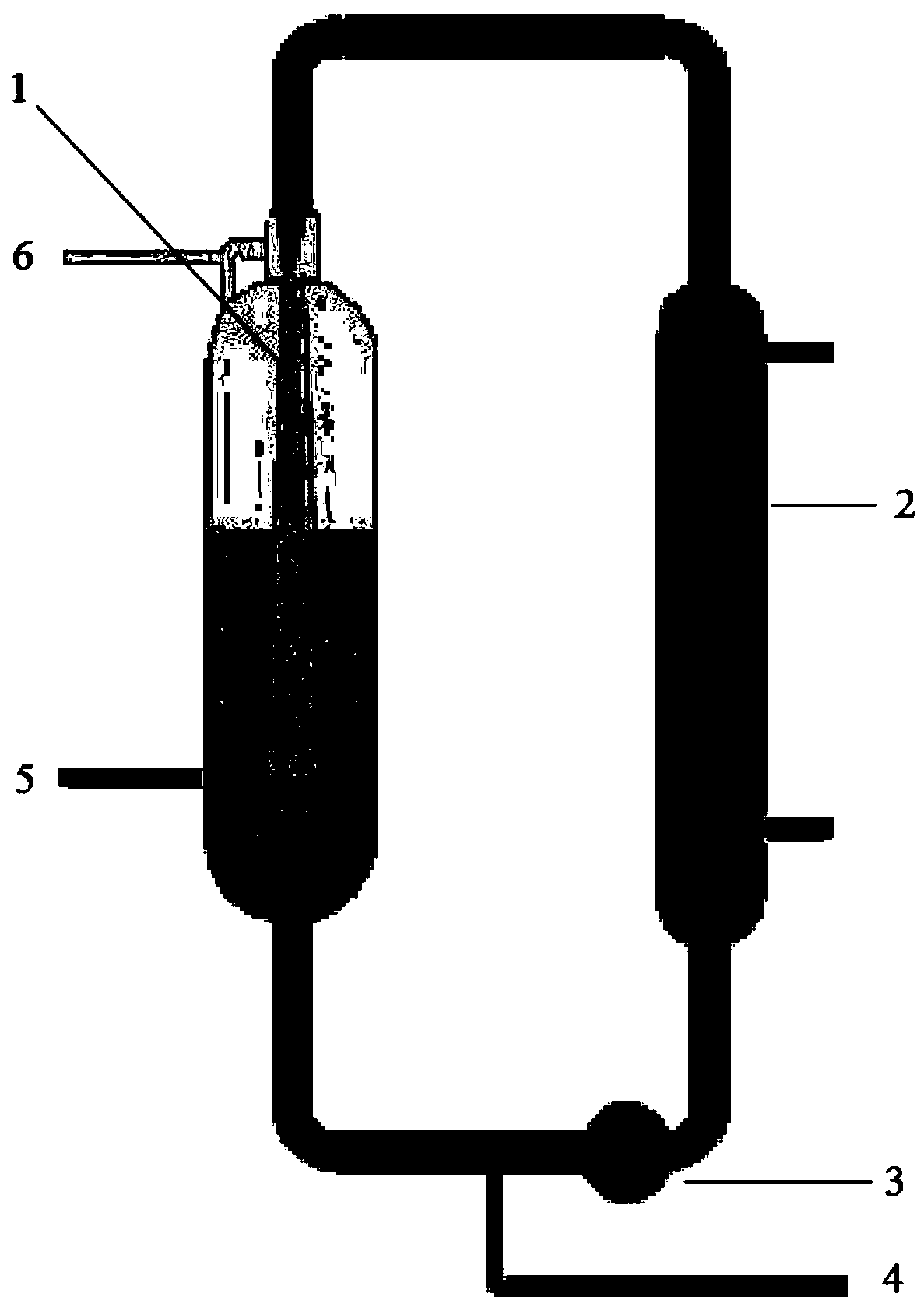
[0029] 1) In a jet loop reactor based on the Venturi effect, the operating principle is based on the Venturi effect, the loop operates as downward flow, and the jet mixer 1 end of the reactor is immersed in the liquid phase of the loop reactor, First carry out nitrogen replacement, then add 10 mol of acrylic acid and 10 mol of 4-cyanopyridine through the material feed port 5 in sequence, and turn on the external circulation pump 3 at 70°C for high-speed jet mixing.
[0030] 2) Control the temperature at 70°C, slowly add 10 mol of hydrogen chloride raw material into the injection loop reactor through the gas raw material feed port 6, and spray and mix through the jet mixer 1 to achieve a sufficient reaction between the materials, and the feeding time is 2 hour, after the addition finished, after the injection loop reactor continued to react for 2 hours, the first step reaction ended;
[0031] 3) After the first step of the reaction is completed, the temperature of the system is...
Embodiment 2
[0038] 1) In a jet loop reactor based on the Venturi effect, the operating principle is based on the Venturi effect, the loop operates as downward flow, and the jet mixer 1 end of the reactor is immersed in the liquid phase of the loop reactor, First carry out nitrogen replacement, then add 15 mol of acrylic acid and 10 mol of 4-cyanopyridine through the material feed port 5 in sequence, and turn on the external circulation pump 3 at 110°C for high-speed jet mixing.
[0039] 2) Control the temperature at 110°C, slowly add 15 mol of hydrogen chloride raw material into the injection loop reactor through the gas raw material feed port 6, and spray and mix through the jet mixer 1 to achieve a sufficient reaction between the materials, and the feeding time is 1 hour, after the feed ended, after the injection loop reactor continued to react for 1 hour, the first step reaction ended;
[0040] 3) After the first step of the reaction is completed, the temperature of the system is lower...
Embodiment 3
[0047] 1) In a jet loop reactor based on the Venturi effect, the operating principle is based on the Venturi effect, the loop operates as downward flow, and the jet mixer 1 end of the reactor is immersed in the liquid phase of the loop reactor, First carry out nitrogen replacement, then add 12 mol of acrylic acid and 10 mol of 4-cyanopyridine sequentially through the material feed port 5, and turn on the external circulation pump 3 at 90°C for high-speed jet mixing.
[0048] 2) Control the temperature at 90°C, slowly add 13 mol of hydrogen chloride raw material into the injection loop reactor through the gas raw material feed port 6, and spray and mix through the jet mixer 1 to achieve a sufficient reaction between the materials, and the feeding time is 1.5 hour, after the feed ended, after the injection loop reactor continued to react for 1.5 hours, the first step reaction ended;
[0049] 3) After the first step of reaction is completed, the temperature of the system is lower...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com