Method for producing 2-chloro-3-cyanopyridine through continuous reaction
A technology for cyanopyridine and cyanopyridine nitrogen oxide, which is applied in the production field of 2-chloro-3-cyanopyridine, can solve the problems of unsolvable production efficiency, low reaction condition control and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
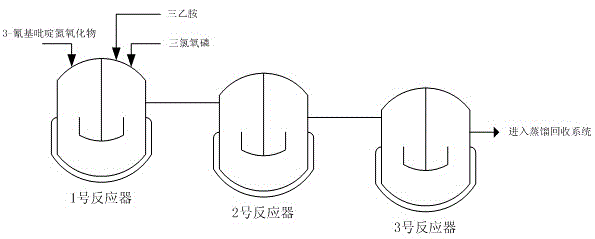
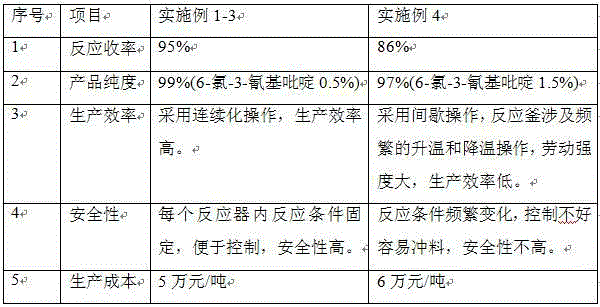
[0014] The weight ratio of 3-cyanopyridine nitrogen oxide, phosphorus oxychloride and triethylamine is 1:5:0.8, and the ratio of 1:5:0.8 is continuously added to the No. 1 reactor, and the temperature in the reactor is maintained at 20°C by controlling the feed rate. ~25°C; the reaction solution enters the No. 2 and No. 3 reactors from the No. 1 reactor in turn, and the temperature of the No. 2 kettle is controlled at 40~50°C, and the temperature of the No. 3 kettle is controlled at 80~90°C. The average residence time of the reaction solution in the No. 3 kettle The time is controlled within 1 to 2 hours, and the reaction liquid in the No. 3 kettle continuously enters the subsequent distillation recovery system.
[0015] The above reaction solution is distilled under reduced pressure to recover phosphorus oxychloride, the vacuum at the end of recovery is -0.06~-0.09MPa, the temperature is 85~105°C, and there is no obvious distillation of phosphorus oxychloride.
[0016] The co...
Embodiment 2
[0018] The weight ratio of 3-cyanopyridine nitrogen oxide, phosphorus oxychloride and triethylamine is 1:5:0.8, and the ratio of 1:5:0.8 is continuously added to the No. 1 reactor, and the temperature in the reactor is maintained at 20°C by controlling the feed rate. ~25°C; the reaction liquid enters the No. 2 reactor from the No. 1 reactor, and the temperature of the No. 2 pot is controlled at 80~90°C. The liquid continuously enters the subsequent distillation recovery system.
[0019] The above reaction solution is distilled under reduced pressure to recover phosphorus oxychloride, the vacuum at the end of recovery is -0.06~-0.09MPa, the temperature is 85~105°C, and there is no obvious distillation of phosphorus oxychloride.
[0020] The concentrated solution after recovery of phosphorus oxychloride is slowly put into 10 times of water to precipitate 2-chloro-3-cyanopyridine, control the discharge temperature not higher than 50°C, cool down to below 40°C after discharge and ...
Embodiment 3
[0022] 3-cyanopyridine nitrogen oxide and phosphorus oxychloride are continuously added to No. 1 reactor at a ratio of 1:5 by weight; the reaction solution overflows from No. 1 reactor and enters No. The weight ratio of pyridine nitrogen oxide and triethylamine is 1:0.8, and triethylamine is continuously added to No. 2 reactor, and the reaction temperature is controlled at 20 ° C ~ 25 ° C; the reaction liquid enters No. 3 reaction tank from No. 2 reactor The temperature of the No. 3 kettle is controlled at 80-90°C. The average residence time of the reaction solution in the No. 3 kettle is controlled at 1-2 hours. The reaction solution in the No. 3 kettle continuously enters the subsequent distillation recovery system.
[0023] The above reaction solution is distilled under reduced pressure to recover phosphorus oxychloride, the vacuum at the end of recovery is -0.06~-0.09MPa, the temperature is 85~105°C, and there is no obvious distillation of phosphorus oxychloride.
[0024] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com