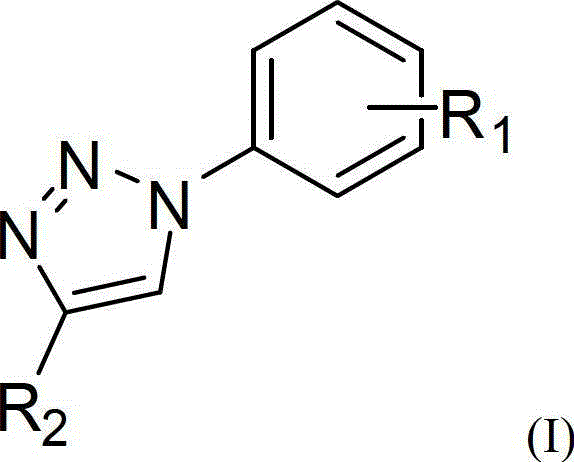
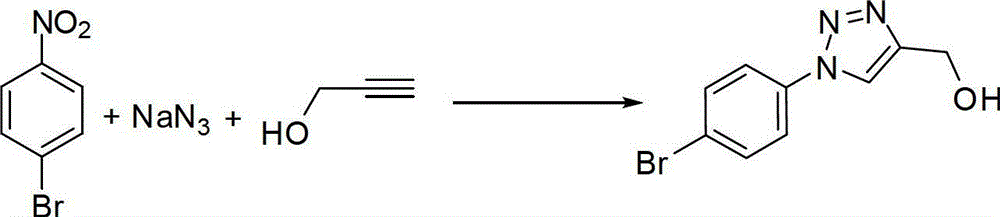One-pot synthetic method for 1,2,3-triazole compounds
A synthesis method and compound technology, applied in the direction of organic chemistry, etc., can solve problems such as the reduction of reaction efficiency, and achieve the effects of high safety, simple operation and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of (1-(4-bromophenyl)-4-hydroxymethyl)-1,2,3-triazole
[0032]
[0033] Add 201mg (1mmol) of 4-bromonitrobenzene, 130mg (2mmol) of sodium azide, 56mg (1mmol) of propynyl alcohol, 40mg (0.2mmol) of sodium ascorbate, and 38mg (0.2mmol) of cuprous iodide to the round bottom flask. ), adding 20mL of hexamethylphosphoric triamide, heating to 60°C, stirring and reacting for 8 hours under the protection of nitrogen. After the reaction was detected by TLC, the insoluble matter was removed by suction filtration, 100 mL of ethyl acetate was added, washed twice with saturated brine, and the organic phase was dried with a desiccant. The desiccant was removed by suction filtration, and the filtrate was spin-dried. The obtained product was purified by silica gel column chromatography to obtain 272 mg of a light yellow solid with a yield of 90.9%.
[0034] The data are characterized as follows:
[0035] 1 H NMR (500MHz, DMSO-d6): δ4.60(s, 2H), 7.78-7.89(m, 4H), 8.73...
Embodiment 2
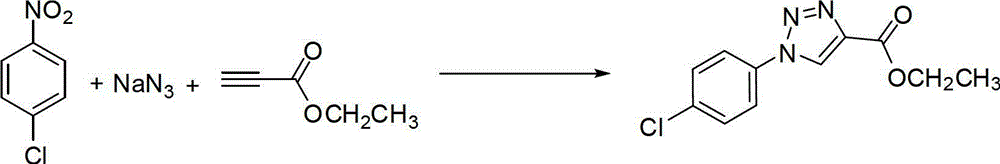
[0037] Preparation of (1-(4-chlorophenyl)-4-ethoxycarbonyl)-1,2,3-triazole
[0038]
[0039] Add 157mg (1mmol) of 4-chloronitrobenzene, 163mg (2.5mmol) of sodium azide, 117mg (1.2mmol) of ethyl propiolate, 99mg (0.5mmol) of sodium ascorbate, and cuprous iodide to the round-bottomed flask. 96mg (0.5mmol), add 30mL hexamethylphosphoric triamide, heat to 100°C, stir and react for 5 hours under nitrogen protection. After the reaction was detected by TLC, the insoluble matter was removed by suction filtration, 100 mL of ethyl acetate was added, washed twice with saturated brine, and the organic phase was dried with a desiccant. The desiccant was removed by suction filtration, and the filtrate was spin-dried. The obtained product was purified by recrystallization to obtain 225 mg of off-white solid, with a yield of 88.2%.
[0040] The data are characterized as follows:
[0041] 1 H NMR(500MHz,DMSO-d6):δ9.51(s,1H),8.03(d,J=8.4,2H),7.70(d,J=8.4,2H),4.36(q,J=7.0,2H ),1.34(t,J=7...
Embodiment 3
[0043] Preparation of (1-(4-ethoxycarbonyl)-4-hydroxymethyl)-1,2,3-triazole
[0044]
[0045] Add 195 mg (1 mmol) of ethyl 4-nitrobenzoate, 260 mg (4 mmol) of sodium azide, 62 mg (1.1 mmol) of propynyl alcohol, 59 mg (0.3 mmol) of sodium ascorbate, and 75 mg of copper sulfate pentahydrate into the round bottom flask (0.3mmol), add 40mL of hexamethylphosphoric triamide, heat to 50°C, stir and react for 10 hours under the protection of nitrogen. After the reaction was detected by TLC, the insoluble matter was removed by suction filtration, 120 mL of ethyl acetate was added, washed twice with saturated brine, and the organic phase was dried with a desiccant. The desiccant was removed by suction filtration, and the filtrate was spin-dried. The obtained product was purified by recrystallization to obtain 209 mg of off-white solid, with a yield of 84.7%. The data are characterized as follows:
[0046] 1H NMR(500MHz,DMSO-d6):δ8.82(s,1H),8.43-7.76(m,4H),4.64(d,J=5.4Hz,2H),4.40(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com