Crystal form of dihydropyrimidine derivative, preparation method of crystal form and application of crystal form in medicine
A crystal form and drug technology, which is applied in the direction of drug combinations, medical preparations containing active ingredients, antineoplastic drugs, etc., can solve problems affecting drug efficacy, stability and pharmacokinetic properties, and inconvenience in preparation development.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
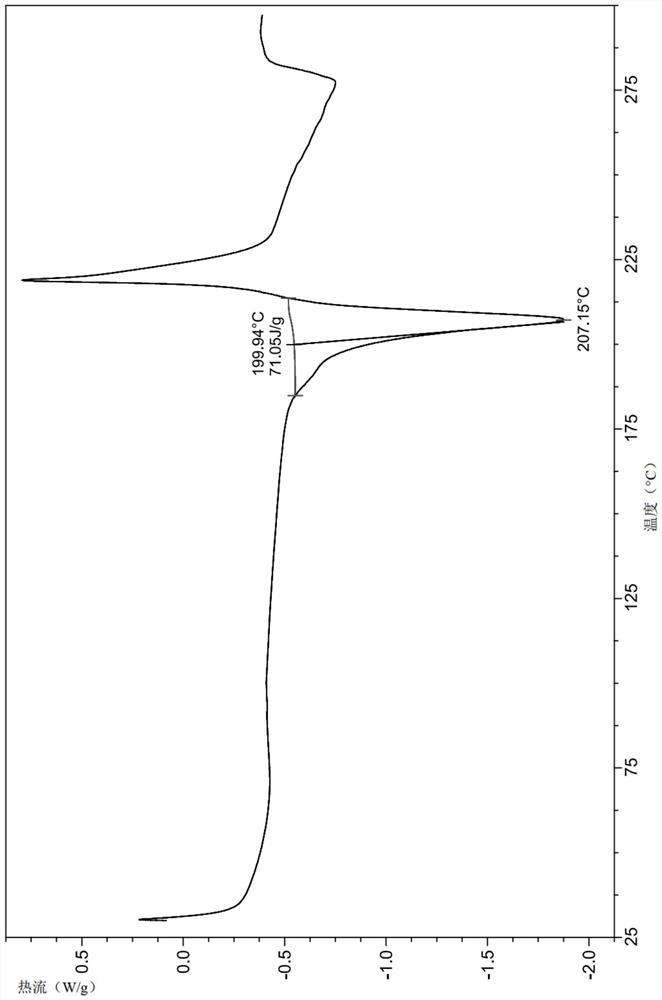
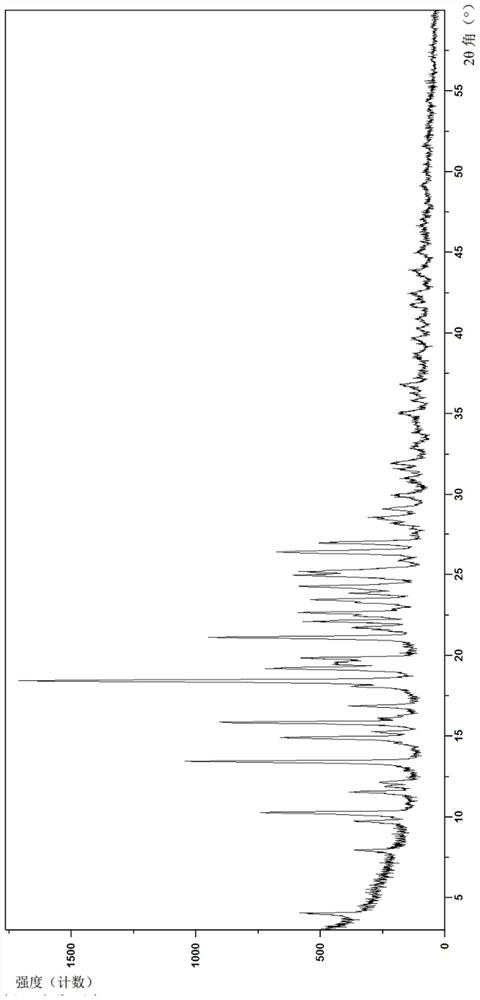
Image
Examples
preparation example Construction
[0116] Preparation of compound represented by formula (I)
[0117] Step 1) Synthesis of Compound 1-1
[0118]
[0119] Drop into acetone (3138kg) in the reactor, add 1,4-bis-Boc-2-piperazinecarboxylic acid (198.74kg, 601.6mol) under stirring, after stirring and dissolving completely, add (S)-1-phenylethylamine again (80.0 kg, 660.2 mol). After the addition, the reaction was stirred at 30±5°C for 18h, centrifuged, and the filter cake was washed with acetone (627.8kg), and the filter cake was vacuum-dried at 60±5°C for 16h to obtain a white solid compound 1-1 (85.32kg, 31.4%) . MS(ESI,pos.ion)m / z:329.3[M-H] - .
[0120] Step 2) Synthesis of Compound 1-2
[0121]
[0122] In the reactor, add water (853.8kg), ethyl acetate (923.0kg) and compound 1-1 (85.22kg) successively, the reaction mixture is stirred at 25 ± 5 ° C, and concentrated hydrochloric acid is added dropwise to adjust pH to 3~4, Set aside to layer. The aqueous layer was extracted with ethyl acetate (460.6...
Embodiment 1
[0146] Example 1 is (4-((S)-7-(((R)-6-(2-chloro-4-fluorophenyl)-5-(methoxycarbonyl)-2-(thiazol-2-yl) )-3,6-dihydropyrimidin-4-yl)methyl)-3-oxohexahydroimidazo[1,5-a]pyrazin-2(3H)-yl)benzoic acid crystal form A-1 , its preparation method is as follows:
[0147] The compound of formula (I) prepared above (6 g, 11.4 mmol) and dichloromethane (100 mL) were successively added to a dry reaction flask, stirred at room temperature to dissolve completely, and the solvent was evaporated under reduced pressure to obtain a foamy solid. Anhydrous methanol (120 mL) was added to the foamy solid, the solid was completely dissolved under stirring, the temperature was raised to reflux, and the system gradually precipitated a solid, kept stirring for 30 min, turned off the heating, cooled to room temperature naturally, then kept stirring for 12 hours, filtered, anhydrous Washed with methanol (20 mL), the solid was dried under vacuum at 50° C. for 8 h to obtain a yellowish solid (5.2 g, 87%).
...
Embodiment 2
[0153] Example 2 is (4-((S)-7-(((R)-6-(2-chloro-4-fluorophenyl)-5-(methoxycarbonyl)-2-(thiazol-2-yl) )-3,6-dihydropyrimidin-4-yl)methyl)-3-oxohexahydroimidazo[1,5-a]pyrazin-2(3H)-yl)benzoic acid crystal form B-1 , its preparation method is as follows:
[0154] The compound of formula (I) prepared above (0.3 g, 0.5 mmol) and dichloromethane (5 mL) were sequentially added to a dry reaction flask, stirred at room temperature to dissolve completely, and the solvent was evaporated under reduced pressure to obtain a foamy solid. Ethyl acetate (5 mL) was added to the foamy solid, the temperature was raised to 70° C., kept stirring for 30 min, the heating was turned off, and the temperature was naturally cooled to room temperature. Stirring was continued for 12 h, filtered, and the filter cake was washed with ethyl acetate (1.5 mL), and then dried under vacuum at 60° C. for 12 h to obtain a yellowish solid (0.27 g, 90%).
[0155] Result identification:
[0156] (1) LC-MS: MS (ESI, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com