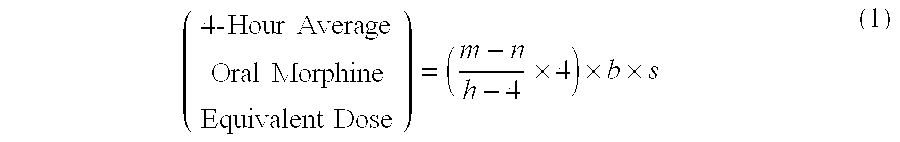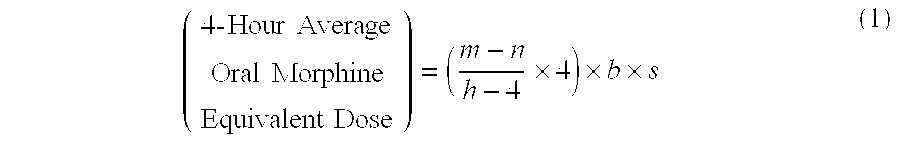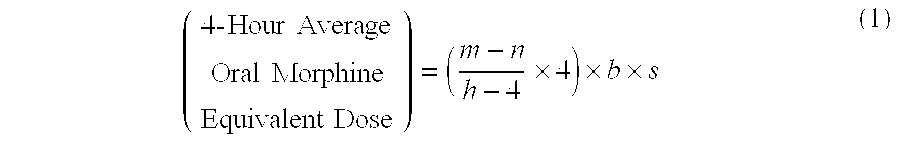Methods of Converting a Patient's Treatment Regimen from Intravenous Administration of an Opioid to Oral Co-Administration of Morphine and Oxycodone Using a Dosing Algorithm to Provide Analgesia
a technology of opioid and dosing algorithm, applied in the field of patient pain treatment, can solve the problems of ineffective pain management, no longer practicable repeated iv dosing, and insufficient synergistic levels of antinociception
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0085]A patient undergoes surgery that is completed at 12:30 PM, whereby IV PCA morphine is started at 1:00 PM. At 7:00 AM the next morning IV PCA administration of morphine is stopped and the patient is converted to oral MOXDUO®, which is a combination of morphine sulfate and oxycodone hydrochloride in the ratio of approximately 3:2 by weight in a form for oral administration for immediate release. Therefore, from the beginning of the IV PCA morphine administration to the end is a total time of 18 hours of IV PCA morphine dosing available for the calculation of the algorithm. During the first four hours of that total time of IV PCA morphine dosing, the patient receives 9 mg of morphine. The patient receives a total amount of 129 mg of morphine during the total time of IV PCA morphine administration. Therefore, the net amount of morphine administered by IV PCA is calculated by subtracting 9 mg (the amount of IV PCA morphine administered during the first four hours of IV PCA) from th...
example 2
[0086]A patient undergoes surgery that is completed at 2:00 PM, whereby IV PCA morphine is started at 2:30 PM. At 7:30 AM the next morning IV PCA administration of morphine is stopped and the patient is converted to oral MOXDUO®, which is a combination of morphine sulfate and oxycodone hydrochloride in the ratio of approximately 3:2 by weight in a form for immediate release. Therefore, from the beginning of the IV PCA morphine administration to the end is a total time of 17 hours of IV PCA morphine dosing available for the calculation of the algorithm. During the first four hours of that total time of IV PCA morphine dosing, the patient receives 9 mg of morphine IV PCA and a nurse gives 2 mg IV morphine through the PCA pump for a total of 11 mg of IV morphine during the first four hours. The patient receives a total amount of 60 mg of morphine during the total time of IV PCA morphine administration. Therefore, the net amount of morphine administered by IV PCA is calculated by subtra...
example 3
[0087]An open-label, multicenter, multiple-dose pilot study of flexible doses of oral MOXDUO® in a 3:2 ratio of morphine sulfate to oxycodone hydrochloride, compared to PERCOCET® (1-2 tablets of 5 mg / 325 mg oxycodone / acetaminophen) for the management of acute, moderate to severe postoperative pain following unilateral total knee arthroplasty or total hip arthroplasty was conducted. One objective of the study was to evaluate the adequacy of an algorithm for conversion of IV PCA morphine to oral morphine-equivalent doses of MOXDUO® administered every 4 to 6 hours over a 48-hour treatment period. Any adverse events, including Treatment-Emergent Adverse Events (TEAE) or Serious Adverse Events (SAE), including signs of abuse potential were also assessed.
Testing Protocol
[0088]Immediate post-operative analgesia consisted of PCA IV morphine. Subjects were connected to a PCA pump within 120 minutes after closure of surgery. Morphine doses (0.5-2.0 mg / dose) were administered by PCA pump with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com