Nanoparticles for photodynamic therapy, x-ray induced photodynamic therapy, radiotherapy, chemotherapy, immunotherapy, and any combination thereof
A nanoparticle, X-ray technology, applied in the field of nanoparticles for photodynamic therapy, X-ray-induced photodynamic therapy, radiation therapy, chemotherapy, immunotherapy and any combination thereof, which can solve the problems of limiting the efficacy of PDT
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0146] According to an exemplary embodiment of the presently disclosed subject matter (further described in the Examples below), Hf-porphyrin NMOFs were prepared and used as PS for PDT against drug-resistant head and neck cancer. Without wishing to be bound by any theory, it is believed that the porphyrin-derived bridging ligands were incorporated into stable and porous UiO (Universitetet I Oslo (named after the Norwegian for University of Oslo). Has suitable morphology and size The NMOF structure can provide multiple advantages over other nanoparticle PDT reagents. First, PS molecules or parts can be sufficiently separated in the NMOF framework to avoid aggregation and self-quenching of excited states. Second, Formation of coordination bonds between porphyrin ligands and heavy metal (e.g., Hf) centers can facilitate the transition between systems to increase the efficiency of ROS generation. Third, the porous NMOF structure can provide a pathway for ROS (e.g., singlet oxygen (...
Embodiment 1
[0238] DBP-Hf-like NMOFs for PDT
[0239] 1.1. Materials and cell lines
[0240] Unless otherwise stated, all starting materials were purchased from Sigma-Aldrich (St. Louis, Missouri, United States of America) and Thermo Fisher Scientific (Waltham, Massachusetts, United States). States of America)) and used without further purification.
[0241] The human head and neck cancer cell line SQ20B (cisplatin-resistant) was kindly provided by Dr. Stephen J. Kron (Department of Molecular Genetics and Cell Biology, University of Chicago, Chicago, USA). In DMEM / F12 (1:1) medium (Gibco, Grand Island, New York, USA) containing 20% fetal bovine serum (FBS, Hyclone, Logan, Utah, USA) Culture cells in.
[0242] Athymic female nude mice (6 weeks, 20-22 g) were provided by Harlan Laboratories, Inc (Dublin, Virginia, USA). The study protocol was reviewed and approved by the University of Chicago Animal Care and Use Committee (IACUC).
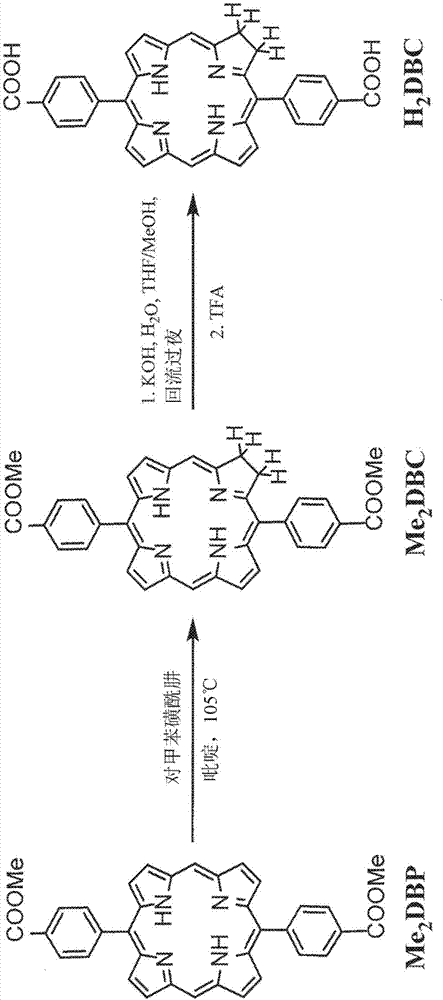
[0243] 1.2. 5,15-bis(p-benzoyloxy)porphyrin (H 2 D...
Embodiment 2
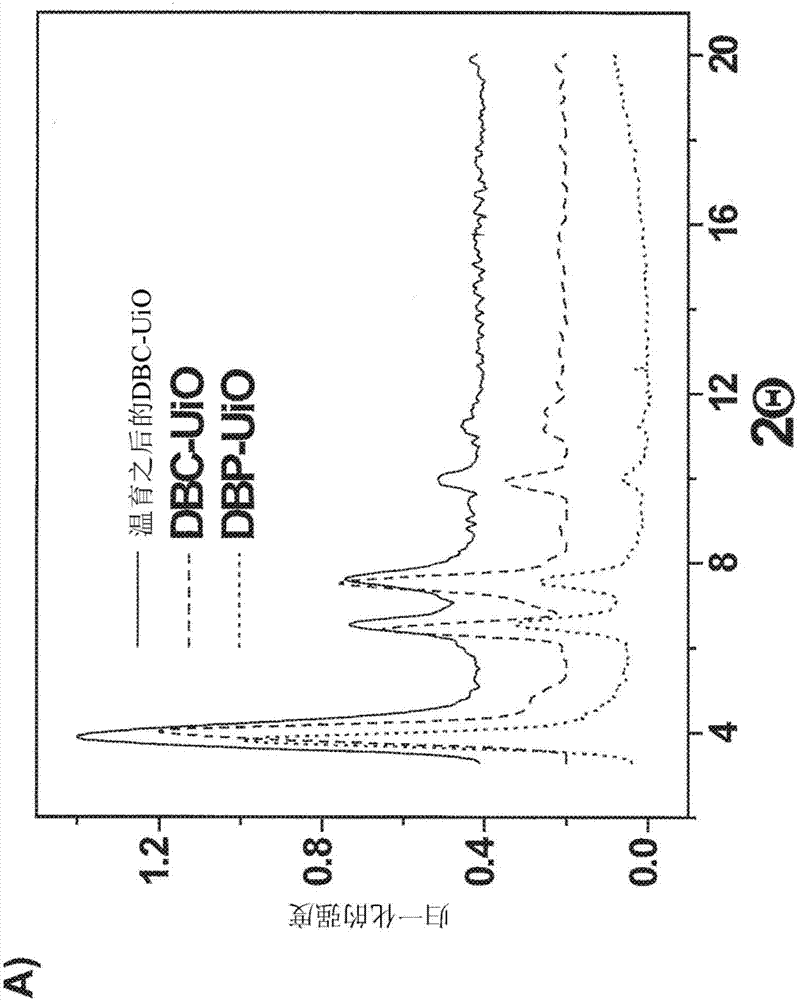
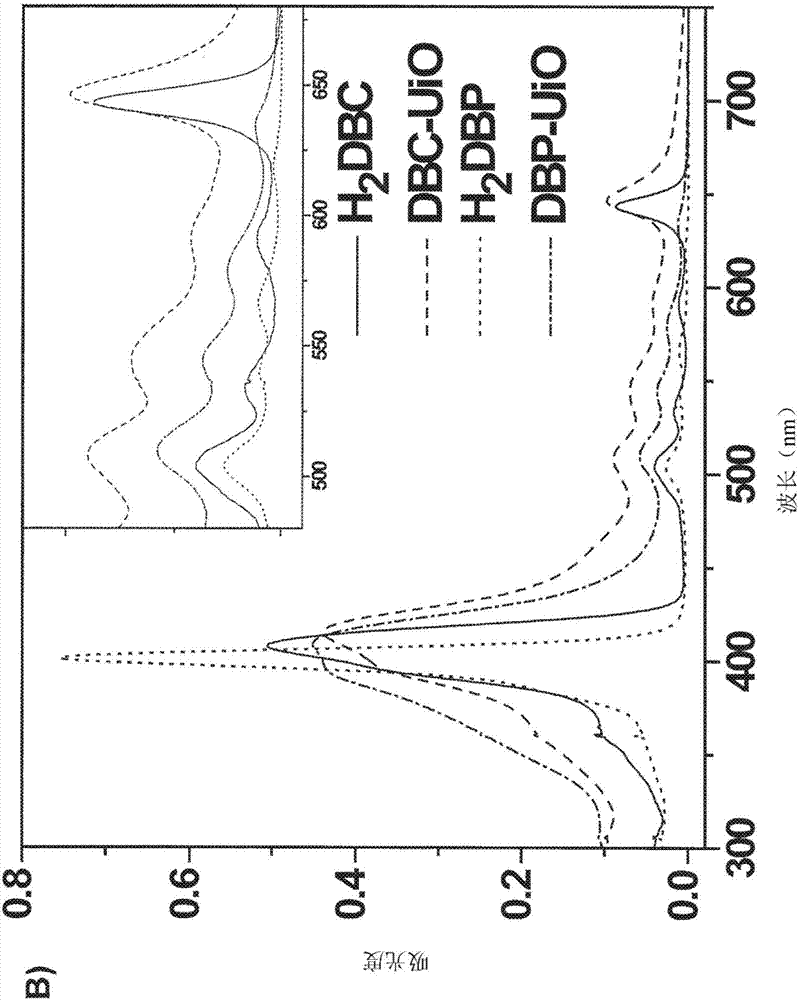
[0291] Overview of the characterization of DBP MOFs
[0292] The porphyrin derivative 5,15-bis(p-benzoyloxy)porphyrin (H 2 DBP), and end with 1 H and 13 CNMR and mass spectral characterization. The linearly arranged dicarboxylate groups of the DBP ligand can be constructed with the framework formula Hf 6 (μ 3 -O) 4 (μ 3 -OH) 4 (DBP) 6 The DBP-UiONMOF. by HfCl 4 and H 2 DBP-UiO was synthesized by solvothermal reaction of DBP in N,N-dimethylformamide (DMF) at 80℃. The resulting dark purple powder was washed sequentially with copious amounts of DMF, 1% triethylamine in ethanol (v / v) and ethanol, and then dispersed in ethanol to form a stock suspension.
[0293] Analogue Zr based on DBP-UiO 6 (μ 3 -O) 4 (μ 3 -OH) 4 (Zn-DPDBP) 6(i.e., Zn-DPDBP-UiO, where DPDBP is 5,15-(p-benzoyloxy)-10,20-diphenylporphyrin), and DPDBP and DBP have similar lengths, and The powder X-ray diffraction (PXRD) pattern of Zn-DPDBP-UiO is similar to that of DBP-UiO. It is believed that DB...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
| The average diameter | aaaaa | aaaaa |
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com