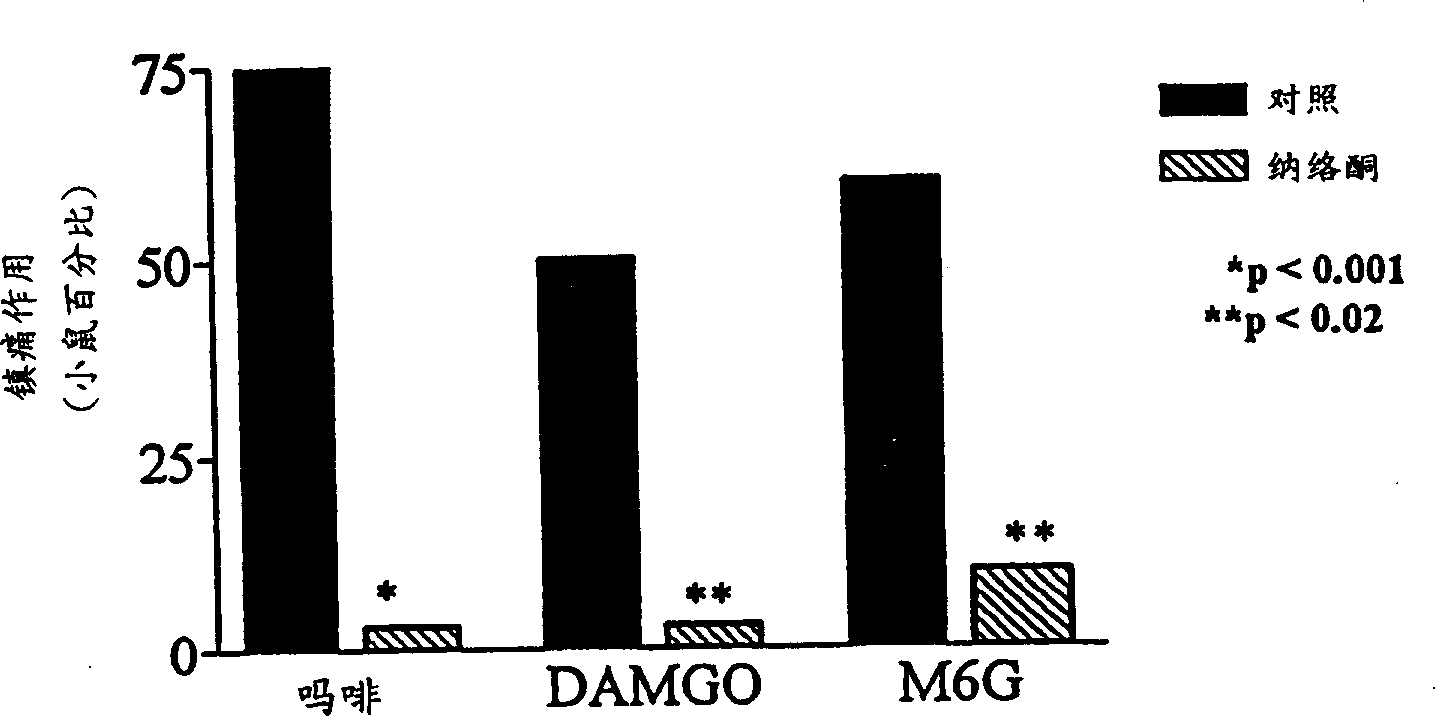Topical compositions comprising opioid analgesic and NMDA antagonist
A technology of opioids and topical drugs, applied in the direction of drug combination, non-central analgesics, active ingredients of heterocyclic compounds, etc., can solve the problem of decreased peripheral efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Materials and methods
[0058] Male Crl:CD-l(ICR)BR mice (25-30 g; Charles River Breeding Laboratories, Bloomington, MA) were housed in a 12-hour light / dark cycle with free access to food and water. Mice were divided into groups of 5 and raised until testing. [ 125 I] NaI (1680 Ci / mmol) was purchased from New England Nuclear (Boston, MA). Morphine, morphine-6β-glucuronide (M6G) and [D-Ala 2 ,MePhe 4 ,Gly(ol) 5 ] Endorphin (DAMGO) was a kind gift from the Research Technologies Branch of National Institute on DrugAbuse (Rockville, MD). MK801 was purchased from Research Biochemicals, Inc. (Natick, MA).
[0059]Systemic administration is administered by subcutaneous (s.c.) injection in the mid-scapular region of the back. As previously reported (Kolesnikov et al., 1996), intracerebroventricular (i.c.v.) and intrathecal administration were performed under mild halothane anesthesia 30 and 15 min before the test, respectively. Injections were performed i.c.v. at a dept...
Embodiment 2
[0063] Topical Morphine and DAMGO Pain Relief
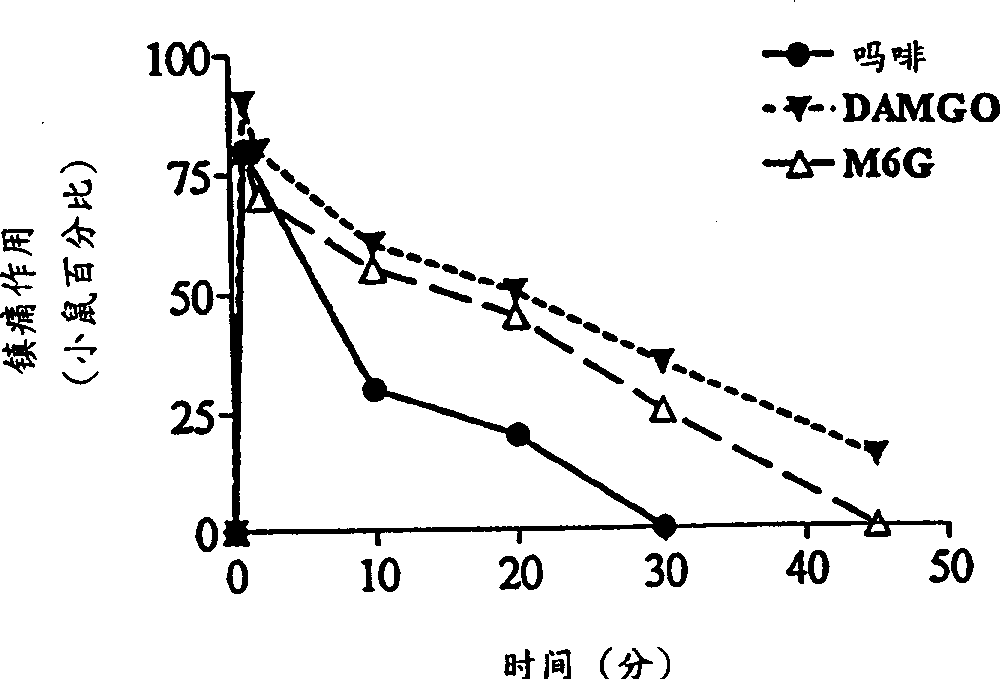
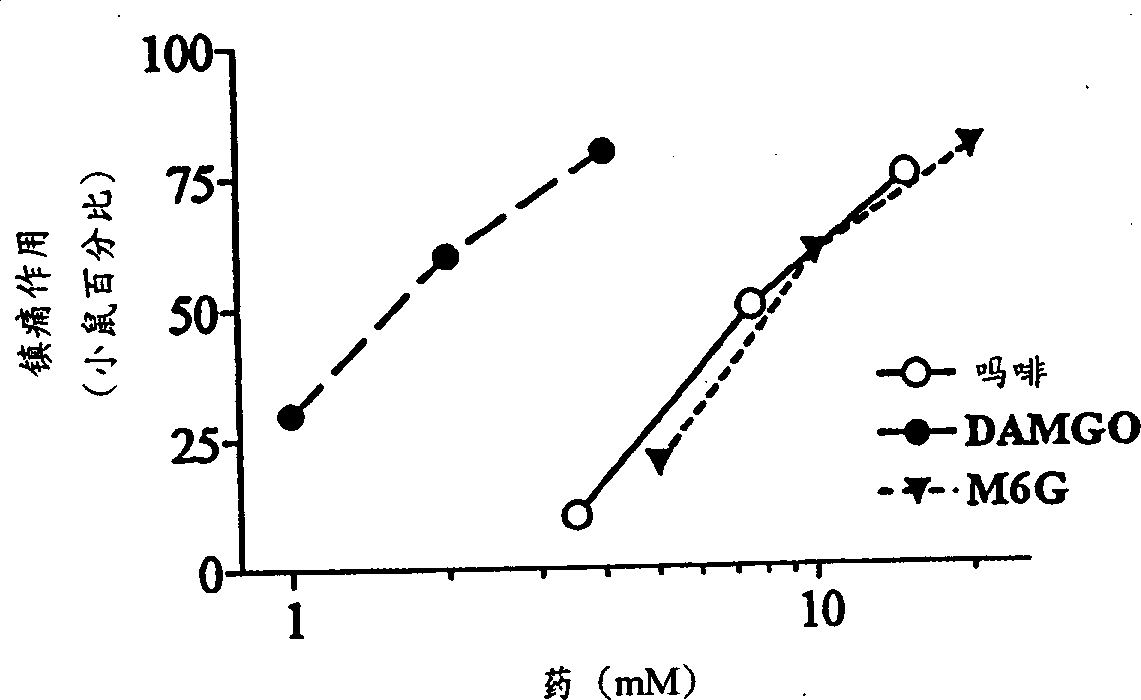
[0064] Our earlier studies demonstrated the potent local analgesic activity of morphine when administered subcutaneously in the tail (Kolesnikov et al., 1996). Morphine is also a strong pain reliever when administered topically. The analgesic response to morphine solution (7.5 mM) gradually increased over time from only 25% after 30 seconds to 50% after 1 minute and 80% after 2 minutes (data not shown). Works very quickly. The analgesic effect can be detected within 1 minute after the mouse tail leaves the opioid solution, and 1 minute is the shortest time detected ( Figure 1a ). However, the duration of the morphine response is relatively short, usually lasting less than 30 minutes. For a fixed exposure time, morphine had a dose-dependent effect ( Figure 1b ;Table 1). In the DMSO solution of μ opioid peptide DAMGO, similar results can also be observed, and its analgesic effect is more than 5 times that of morphine ( Fig...
Embodiment 3
[0072] Analgesic effect of topical morphine-6β-glucuronide
[0073] Morphine-6β-glucuronide (M6G), administered locally by tail subcutaneous injection, was an analgesic but had a 30% ceiling effect at doses of 10 or 30 μg (data not shown). In topical examples, M6G produced a complete analgesic effect with a peak effect immediately after leaving the drug solution ( Figure 1a ), which is similar in potency to morphine ( Figure 1b ;Table 1). Similar to morphine, the proximal tail segment did not produce analgesic effects, and the effects of M6G were easily reversed by systemically administered naloxone ( Figure 2a ). After topical administration, the duration of action of M6G was similar to that of DAMGO and longer than that of morphine ( Figure 1a ). The selective M6G antagonist 3-methoxynaltrexone (3MeONtx) (Brown et al., 1997) also significantly reduced the M6G response ( Figure 2b ). In contrast, the same 3MeONtx dose was not active against the analgesic effects of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com