Antimicrobial articles produced by additive manufacturing
A technology of products and antibiotics, applied in the field of implantable medical devices, which can solve the problems of limited drug load limit and weakening of curative effect over time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]Polylactic acid (PLA) particles were from NatureWorks LLC (Blair, NE, USA). Polycaprolactone (PCL) particles (CAPA™ 6500) were from Plastics Systems Inc. (Lakewood, WA, USA). Vancomycin and gentamicin were from Gold Biotechnology (St. Louis, MO, USA). A PLA blend containing approximately 5% vancomycin was prepared by dry blending 0.28 kg of vancomycin with a 5.8 Kg batch of PLA granules. A PLA blend containing about 2% vancomycin was prepared by dry blending 0.122 kg of vancomycin with a 5.8 Kg batch of PLA granules. A PCL blend containing about 5% gentamicin was prepared by dry blending 0.125 Kg of gentamicin with a 2.5 Kg batch of PCL granules. A PCL blend containing about 2% gentamicin was prepared by dry blending a PCL blend containing about 5% gentamicin with additional PCL to adjust the gentamicin content to about 2%.
[0029] use HiQ system (SINTERSTATION and SLS are registered trademarks of 3D Systems Inc., Valencia, CA, USA), from each batch of polymer / an...
Embodiment 2
[0049] The selected tensile properties of antibiotic-containing plastic Type IV dumbbell test specimens were determined according to the test method set forth in ASTM D638-08 document entitled "Standard Test Method for Tensile Properties of Plastics". Physical Properties, the test methods are published by ASTM International (International Society for Testing and Materials) and are publicly available from their web site at http: / / www.astm.org / Standards / D638.htm. The physical properties of the SLS-printed plastic discs containing antibiotics are listed in Tables 1–4.
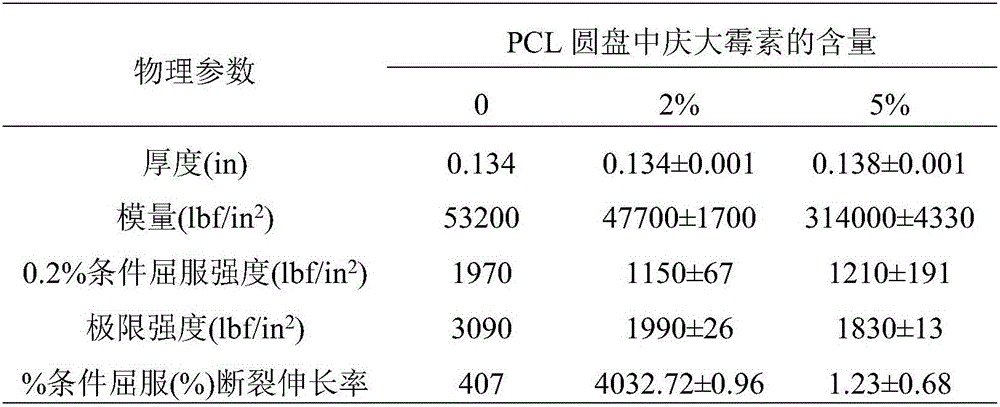
[0050] Table 1: Physical properties of Type IV dumbbell-shaped samples printed with PCL / gentamicin dry blend*
[0051]
[0052] *Data are the mean ± SD of three replicates
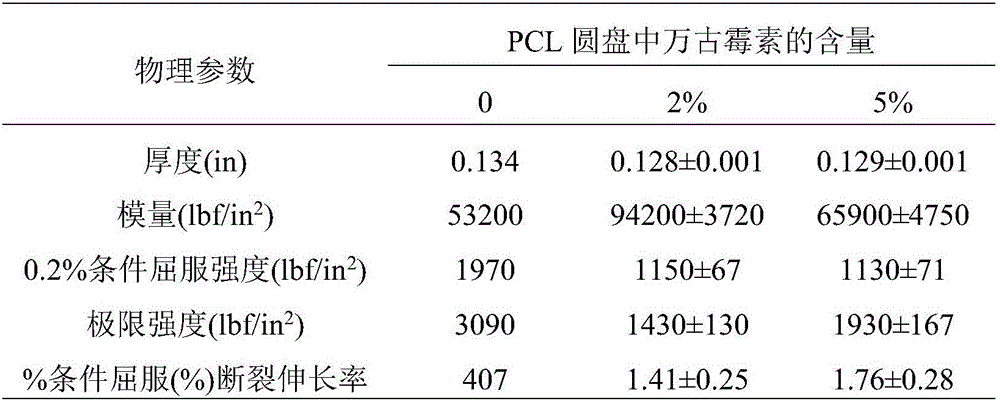
[0053] Table 2: Physical properties of type IV dumbbell-shaped samples printed with PCL / vancomycin dry blend*
[0054]
[0055] *Data are the mean ± SD of three replicates
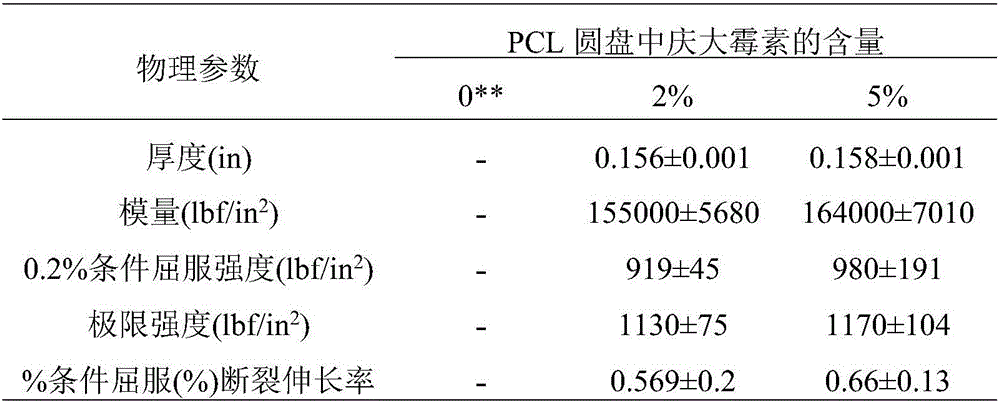
[0056] Table 3: Physical properties of Type IV dumbbell-shap...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com