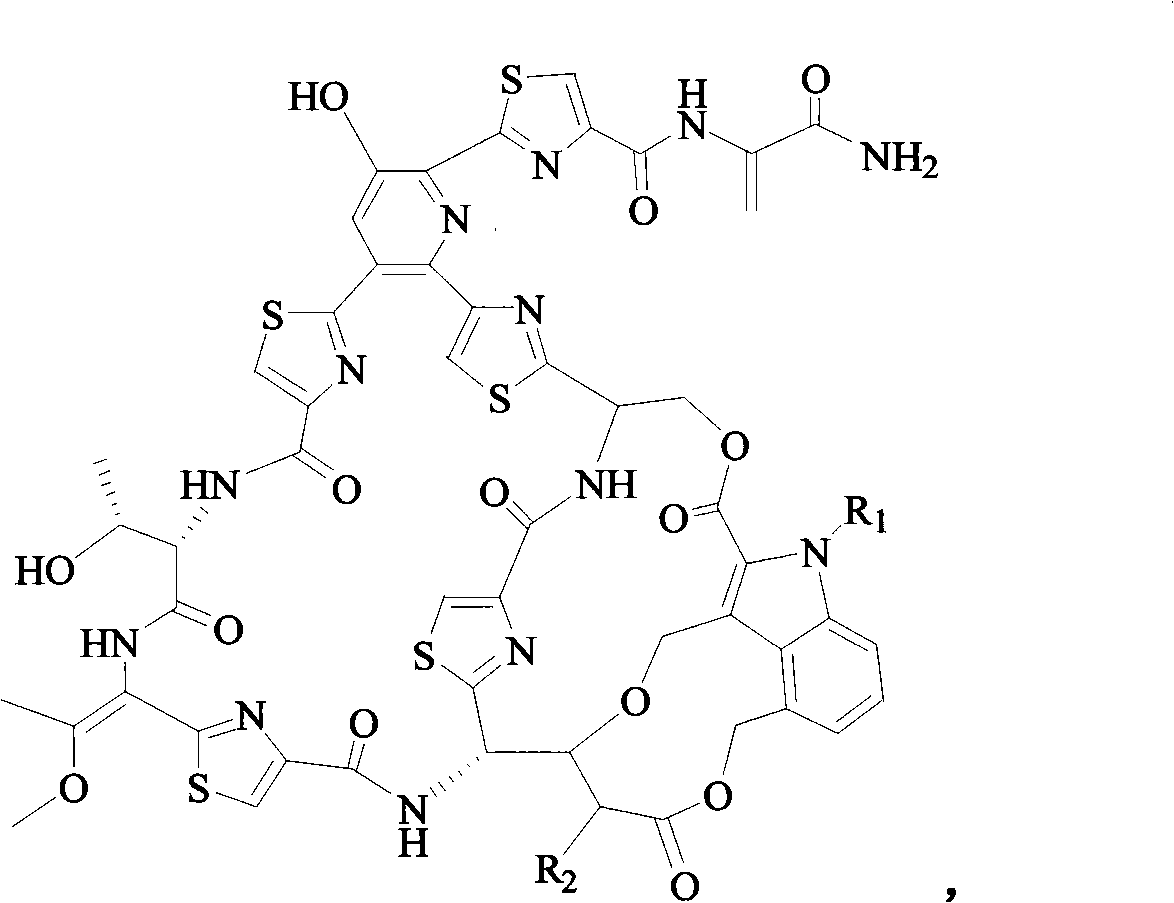
Pharmaceutical composition containing nocathiacin antibiotics
A composition and antibiotic technology, applied in the direction of antibacterial drugs, drug delivery, pharmaceutical formulations, etc., can solve the problems of complex preparation safety, limited medicinal value, poor water solubility, etc., and achieve simple and safe equipment and high cost , the effect of fewer process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment one: the solution containing latent solvent.
[0026] prescription:
[0027] Nocafloxacin I 10mg;
[0028] PEG 400 500 μl;
[0029] 0.9% sodium chloride solution (pH 7.4) 500 μl.
[0030]Process: add the prescription amount of nocafloxacin I, 0.9% sodium chloride solution and PEG400 in sequence, vortex and oscillate for 10 minutes, then ultrasonicate for 30 minutes, and centrifuge to remove the insoluble nocafloxacin I to obtain clear and transparent Solution; the solubility of nocafloxacin I is 6.41 mg / ml and the volume fraction of PEG 400 is 50% as detected by high performance liquid chromatography; the solution is diluted 500-1000 times with 0.9% sodium chloride solution and there is no precipitation within 72 hours Precipitate.
Embodiment 2
[0031] Embodiment two: the solution containing latent solvent.
[0032] prescription:
[0033] Nocafloxacin I 10mg;
[0034] PEG 600 400 μl;
[0035] 5% glucose solution (pH 7.4) 600 μl.
[0036] Process: Add the prescribed amount of nocafloxacin I, 5% glucose solution and PEG600 in sequence in the container, vortex for 10 minutes to mix well, then ultrasonicate for 30 minutes, and centrifuge to remove the insoluble nocafloxacin I to obtain a clear and transparent solution agent; the solubility of nocafloxacin I was 7.54mg / ml and the volume fraction of PEG 600 was 40% as detected by high performance liquid chromatography; no precipitation occurred within 72 hours after the solution was diluted 500-1000 times with 5% glucose solution.
Embodiment 3
[0037] Embodiment three: the solution containing solubilizing agent
[0038] prescription:
[0039] Nocafloxacin I 10mg;
[0040] Poloxamer 20mg;
[0041] 5% glucose solution (pH 7.4) 1000 μl.
[0042] Process: add the prescribed amount of nocafloxacin I, poloxamer and 5% glucose solution to the container in turn, vortex and shake for 10 minutes to mix well, then ultrasonicate for 30 minutes, and centrifuge to remove the insoluble nocafloxacin I to obtain a clear Transparent solution; the solubility of nocafloxacin I detected by high performance liquid chromatography is 2.955 mg / ml; the solution is diluted 500-1000 times with 5% glucose solution and no precipitation occurs within 72 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com