Controlled release of vegetarian pore-sealing capsule in stomach, duodenum, jejunum, ileum and colon
A technology for controlled release and sealing of capsules, which can be used in capsule delivery, medical preparations with non-active ingredients, non-active ingredients of polymer compounds, etc., which can solve the problems of limitation, cost and development difficulties, waste of research and development time and labor costs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] In order to make the narration of the present disclosure more detailed and complete, the following provides an illustrative description of the embodiments and specific embodiments of the present invention; but this is not the only form of implementing or using the specific embodiments of the present invention. The implementations disclosed below can be combined or substituted for each other when beneficial, and other implementations can also be added to one implementation, without further description or illustration.
[0029] In the following description, many specific details will be elaborated so that readers can fully understand the following embodiments. However, embodiments of the invention may be practiced without these specific details. In other instances, well-known structures are shown schematically in order to simplify the drawings.
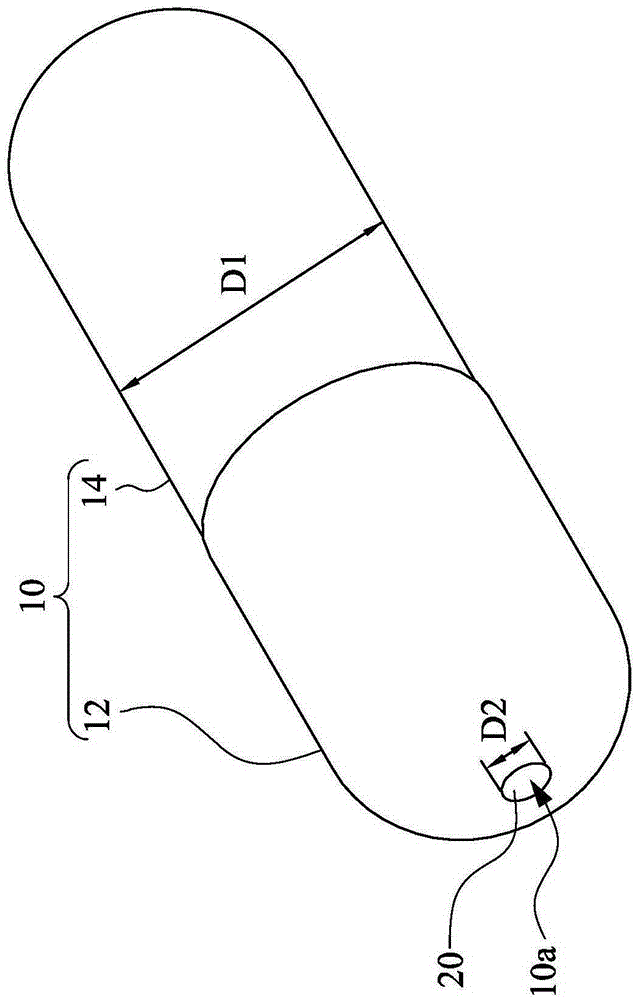
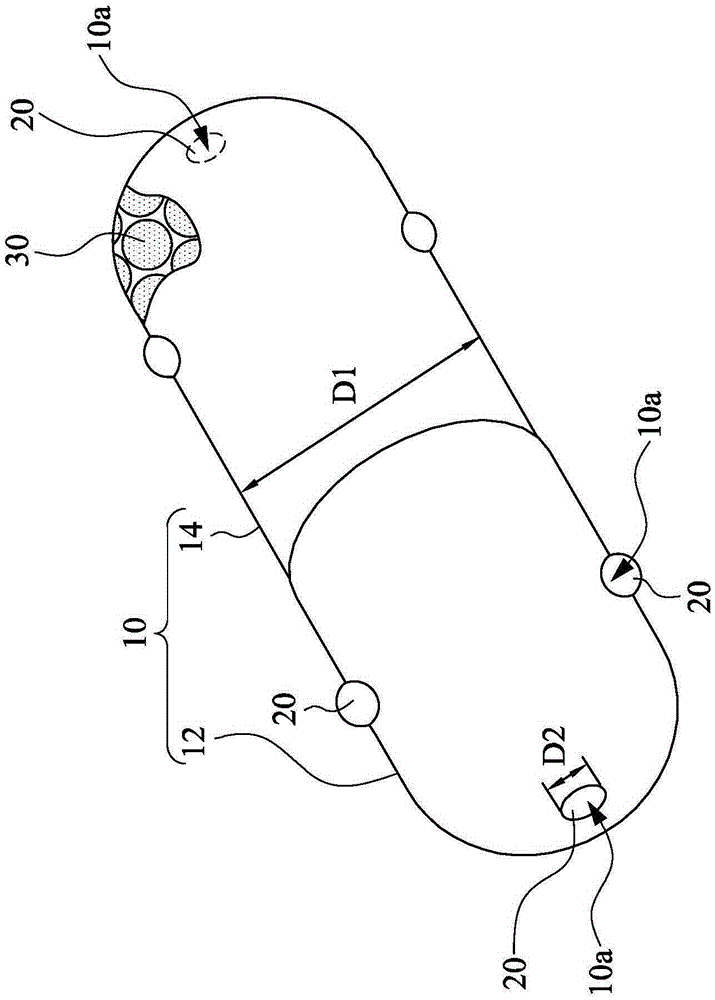
[0030] The invention provides a sealed capsule for gastrointestinal tract controlled release. figure 1 is a schematic diagram...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com