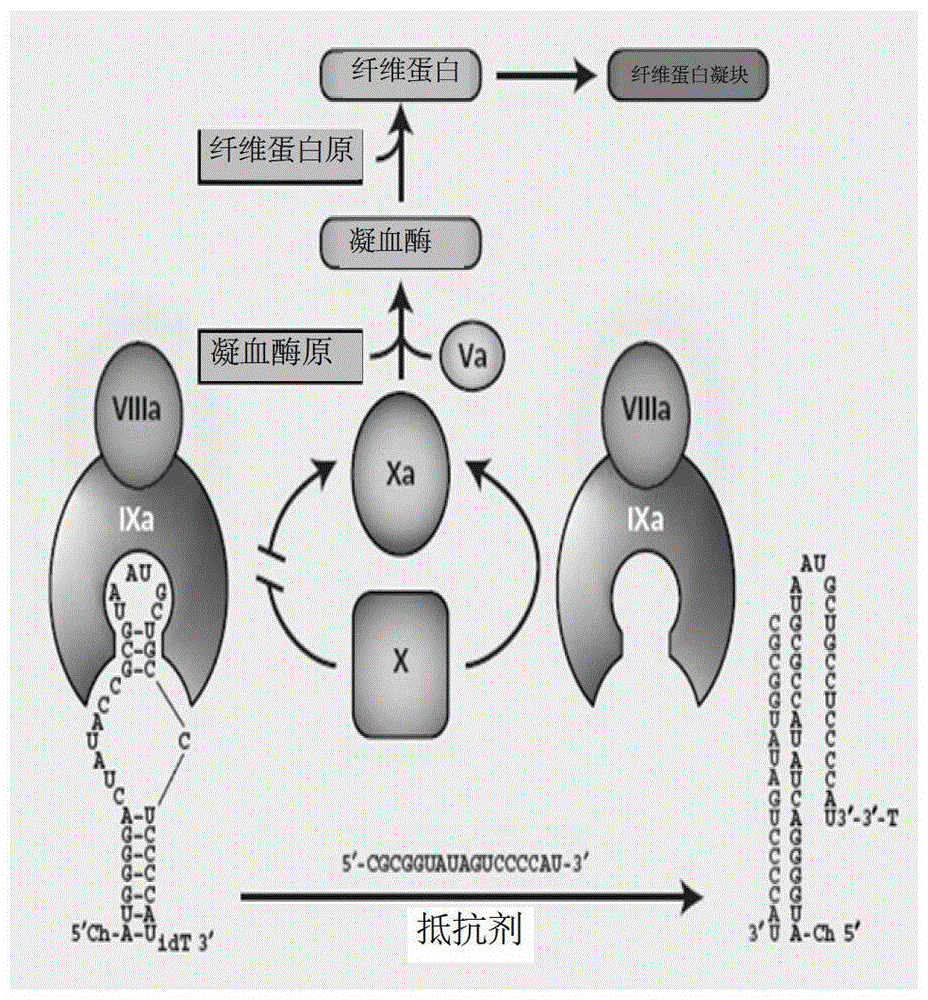
A kind of isothymidine modified thrombin nucleic acid aptamer and its preparation method and application
A technology of nucleic acid aptamer and thrombin, which is applied in the field of biomedicine, can solve problems such as difficult quality control and side effects that are difficult to overcome, and achieve the effects of improving anticoagulant effect, enzyme stability, and in vitro anticoagulant activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1. Solid phase synthesis of isothymidine-incorporated DNA
[0058] DNA was synthesized using an Appllied Biosystems model394 DNA solid-phase synthesizer. Normal deoxynucleoside phosphorylated monomers (dT,dG Ac ) purchased from Shanghai Gemma Pharmaceutical Technology Co., Ltd., CPG (CPG-dG), CAP-A and CAP-B, oxidation I 2 Liquid, Cl 3 CCOOH was purchased from Beijing Aoke Biotechnology Company. 5-Ethylmercapto 1H-tetrazolium solution was purchased from China Pharmaceutical Research and Development Center Co., Ltd. (Beijing).
[0059] According to the method of literature (HW Yu, LR Zhang, JC Zhuo, LT Ma, LH Zhang, Bioorg.Med.Chem., 1996,40,609-614), the isothymidine compound shown in chemical formula I and / or chemical formula II is respectively Prepare isothymidine phosphoramidite monomer shown in chemical formula III and / or chemical formula IV.
[0060]
[0061] Synthesis scale: ~1.0μmol
[0062] Preparation of nucleoside phosphorylation monomer solut...
Embodiment 2
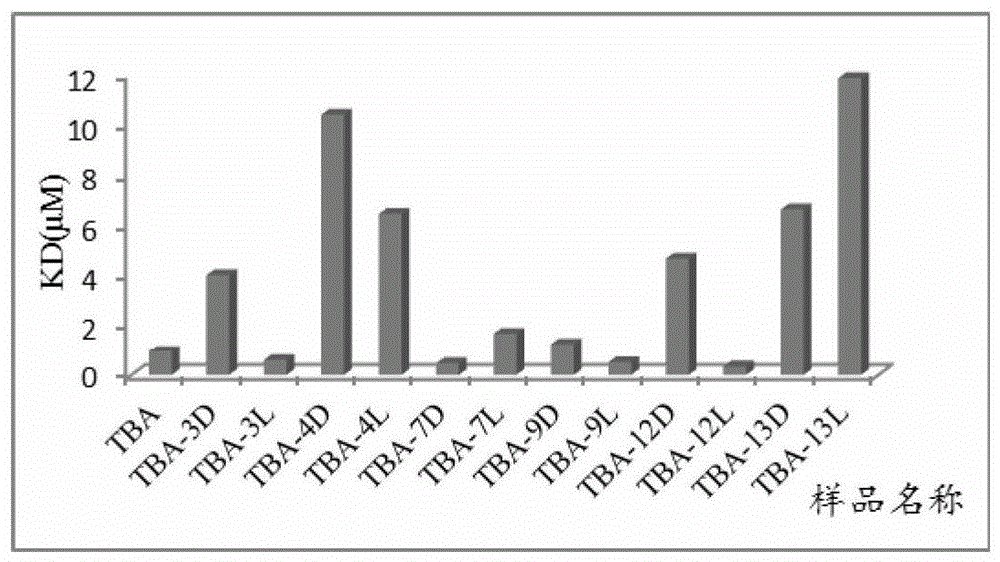
[0073] Example 2. Isothymidine-modified TBA protein level activity test
[0074] TBA-3L: Incorporate isothymidine represented by formula I at the 3-position of TBA sense strand instead of natural isothymidine
[0075] TBA-3D: Incorporate isothymidine represented by formula II at the 3-position of TBA sense strand instead of natural isothymidine
[0076] TBA-4L: Incorporate isothymidine represented by formula I at the 4-position of TBA sense strand instead of natural isothymidine
[0077] TBA-4D: Incorporate isothymidine represented by formula II at the 4-position of TBA sense strand instead of natural isothymidine
[0078] TBA-7L: Incorporate isothymidine represented by formula I at position 7 of the sense strand of TBA instead of natural isothymidine
[0079] TBA-7D: Incorporate isothymidine represented by formula II at position 7 of the sense strand of TBA instead of natural isothymidine
[0080] TBA-9L: Incorporate isothymidine represented by formula I at position 9 of t...
Embodiment 3
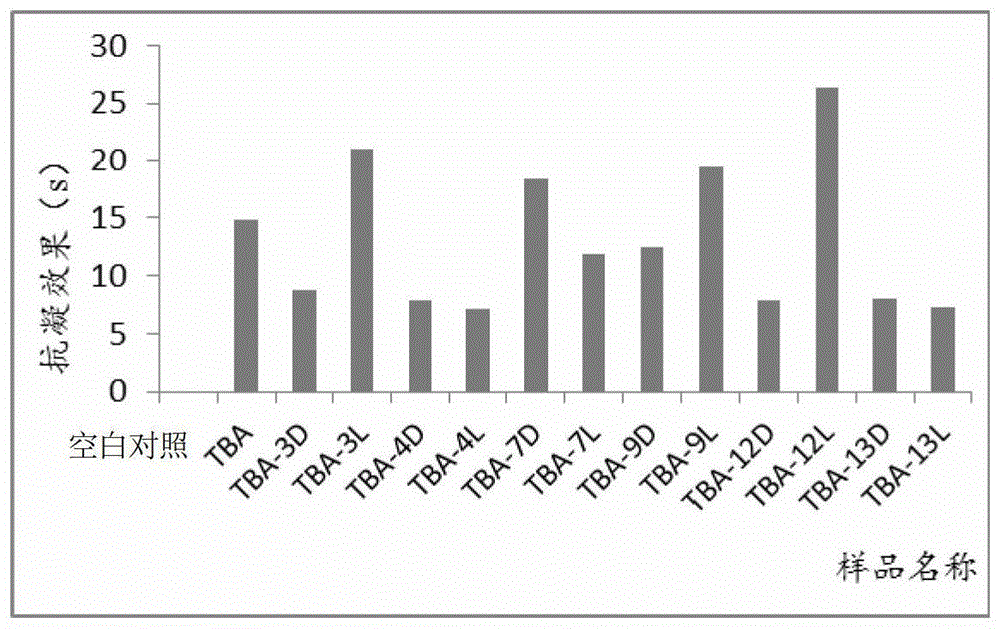
[0089] Example 3. In vitro anticoagulant activity test of isothymidine-modified TBA
[0090] The in vitro anticoagulant activity of isothymidine-modified TBA was determined by PUN-2048B dual-channel coagulation instrument (Beijing Pulang Medical Technology Co., Ltd.). Referring to the requirements of experiments in relevant literature, the concentration of the thrombin nucleic acid aptamer was selected as 0.33 μM. See the result image 3 . Through experiments, it can be found that the anticoagulant effect of TBA-3L, TBA-7D, TBA-9L, and TBA-12L is significantly improved after interacting with human plasma, which is consistent with the previous experimental results on enzyme levels and verifies the validity of the experimental results. reliability. At the same experimental concentration, the anticoagulant time of TBA-12L is about twice that of natural TBA, which can greatly inhibit the activity of thrombin. In vitro experiments show that TBA-12L is a promising new high-speci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com