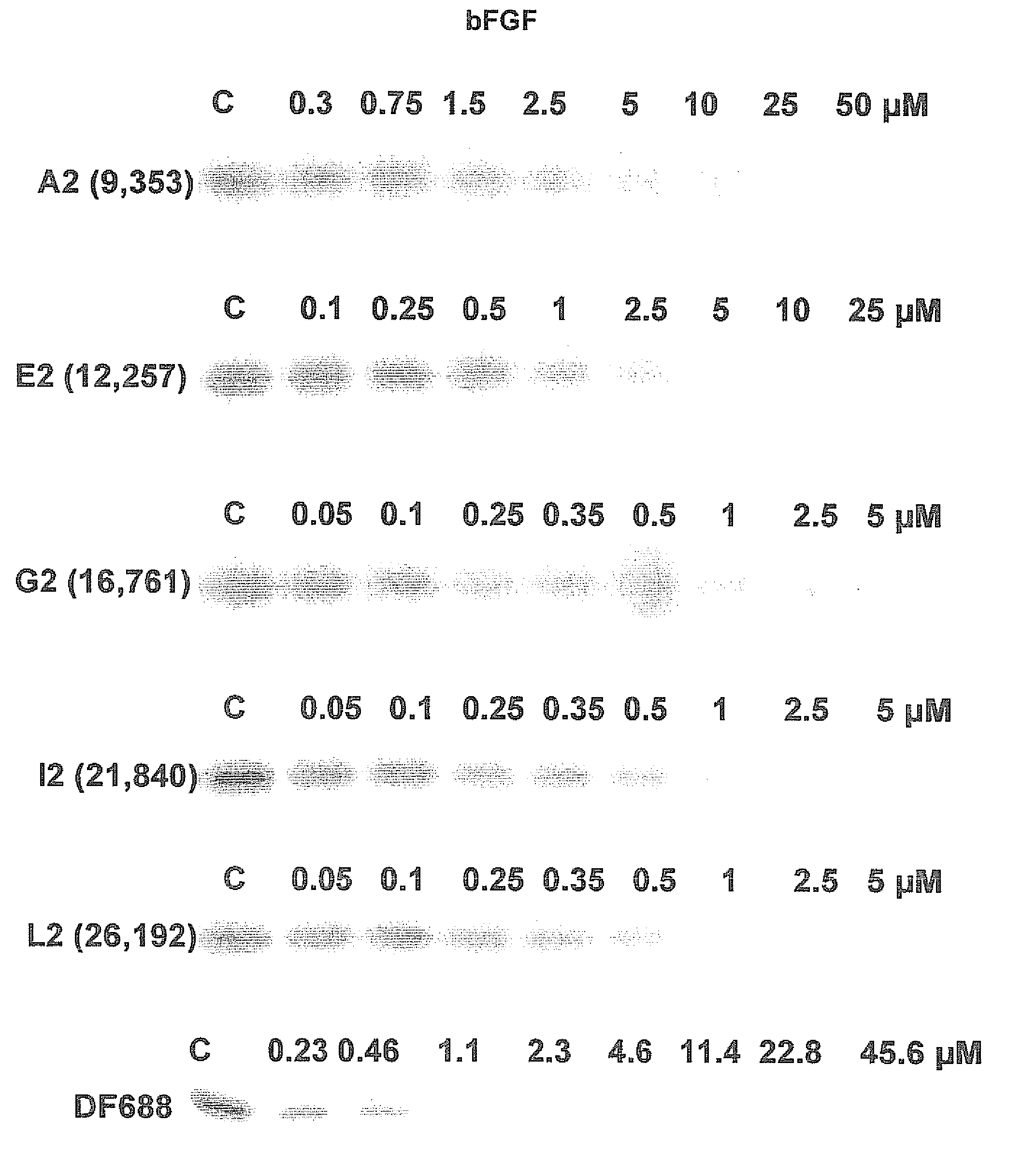
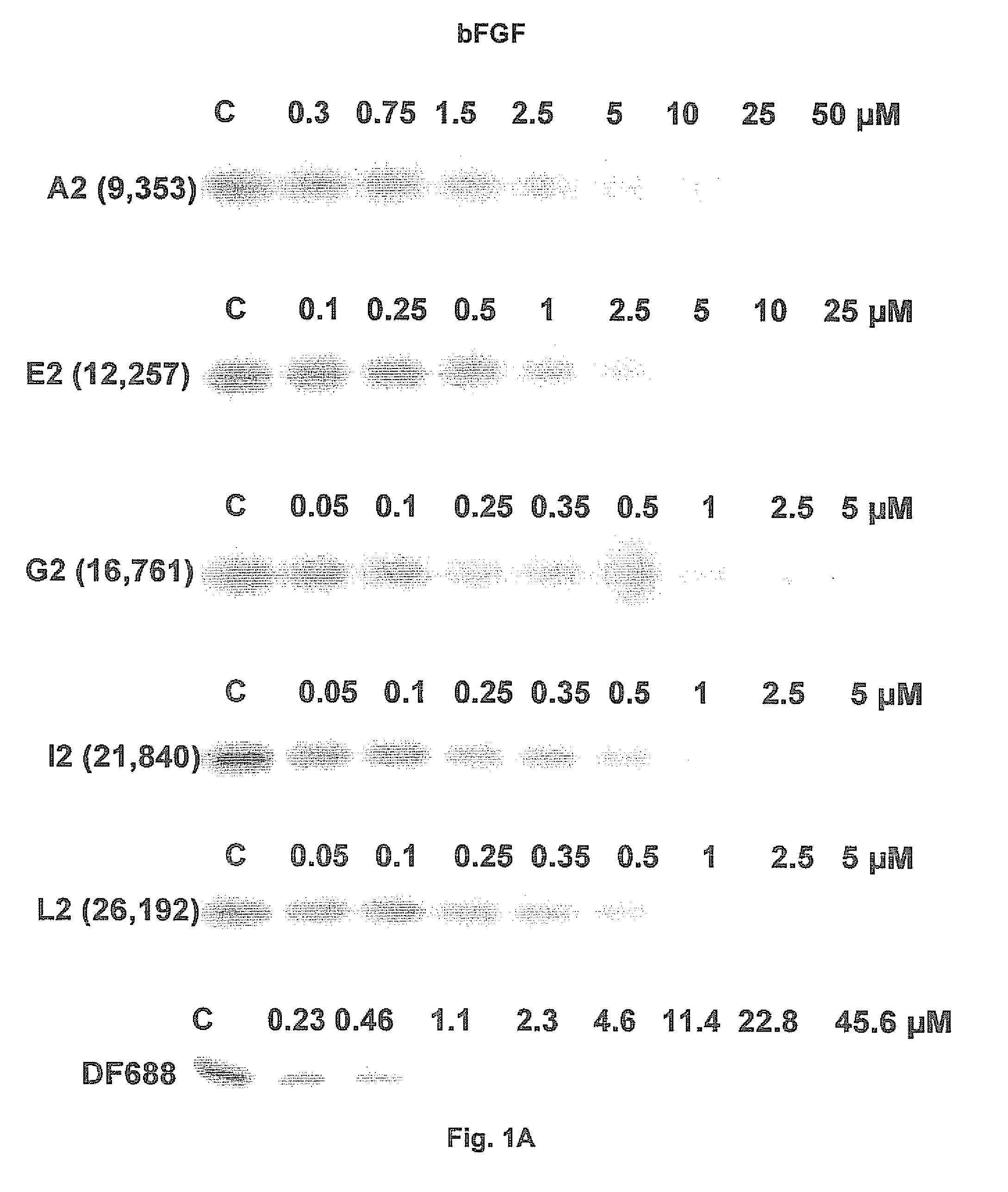
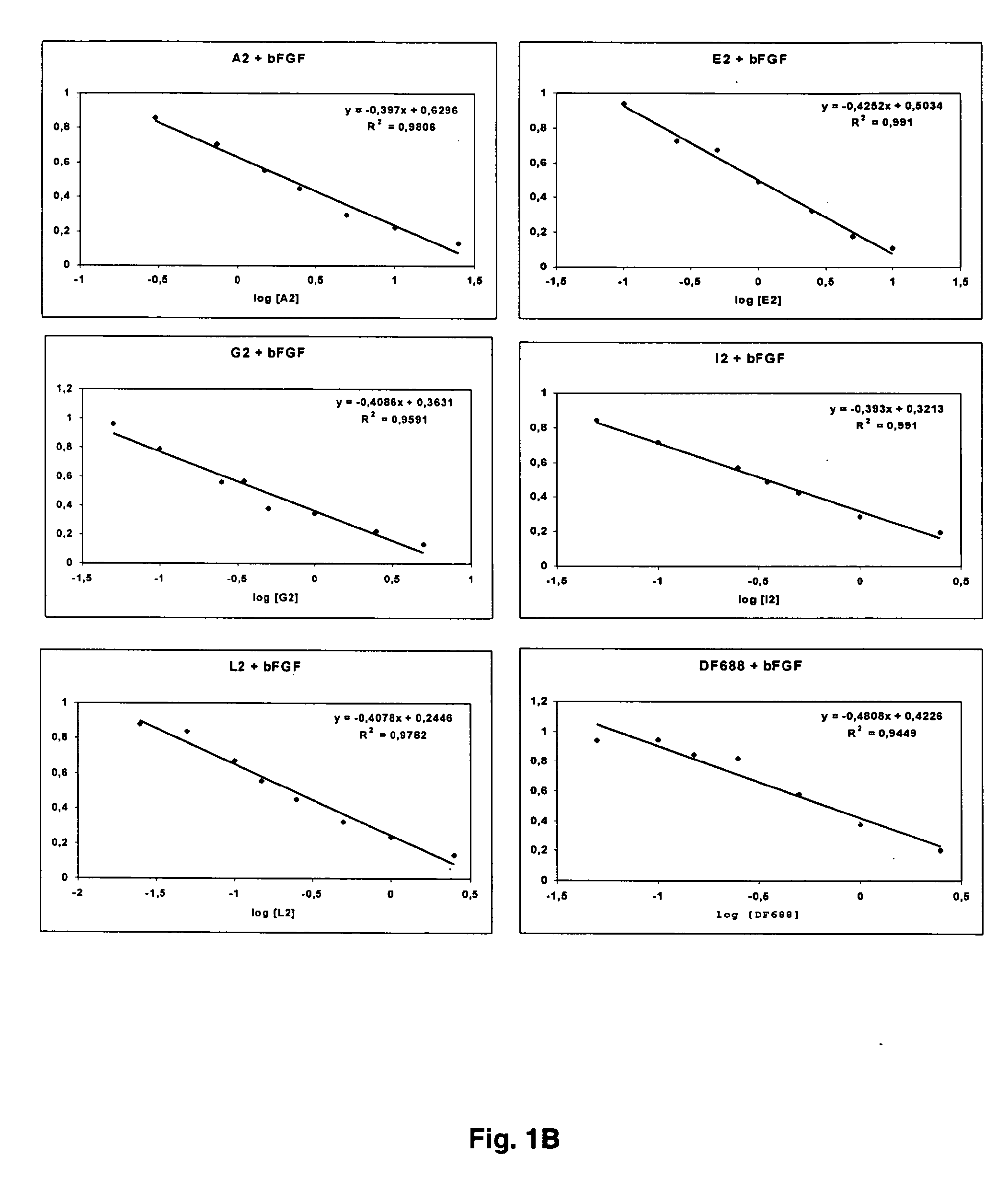
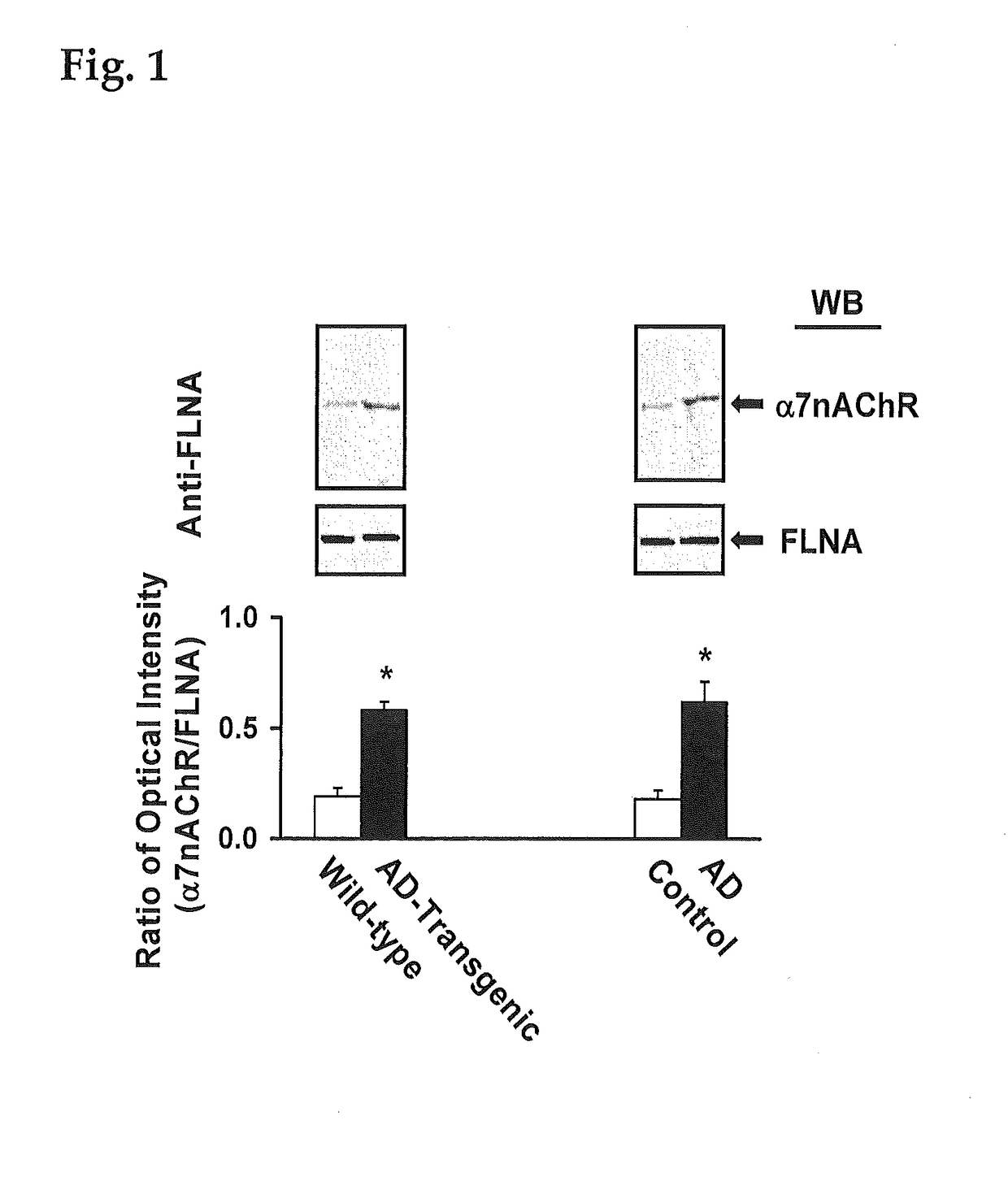
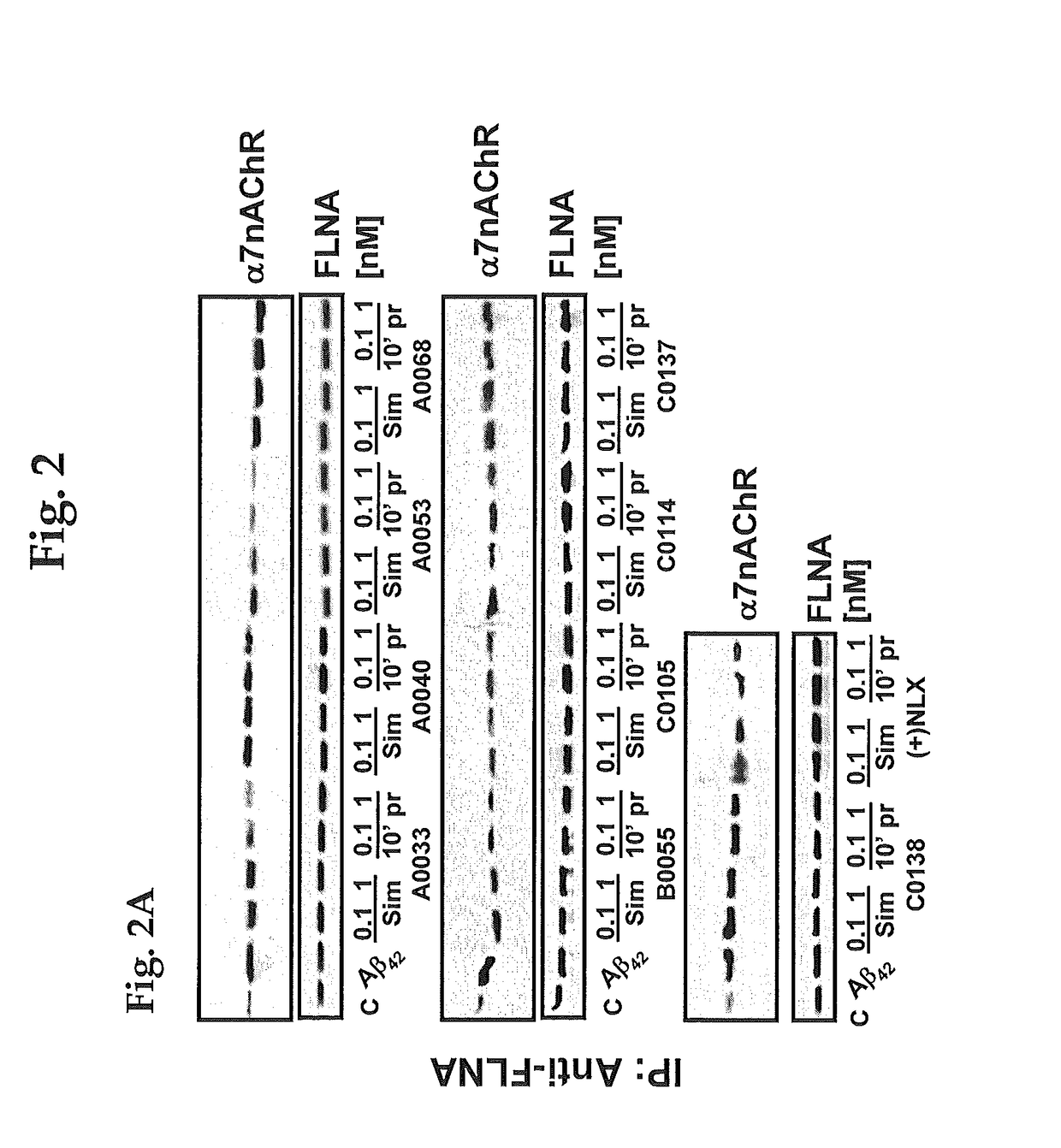
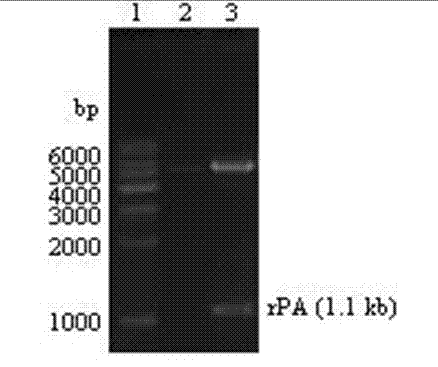
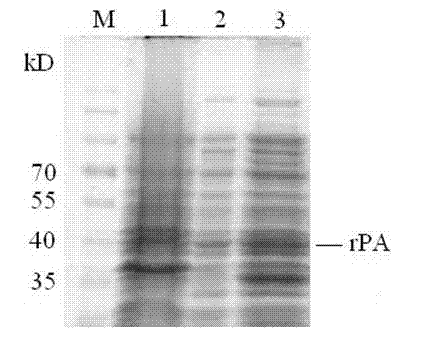
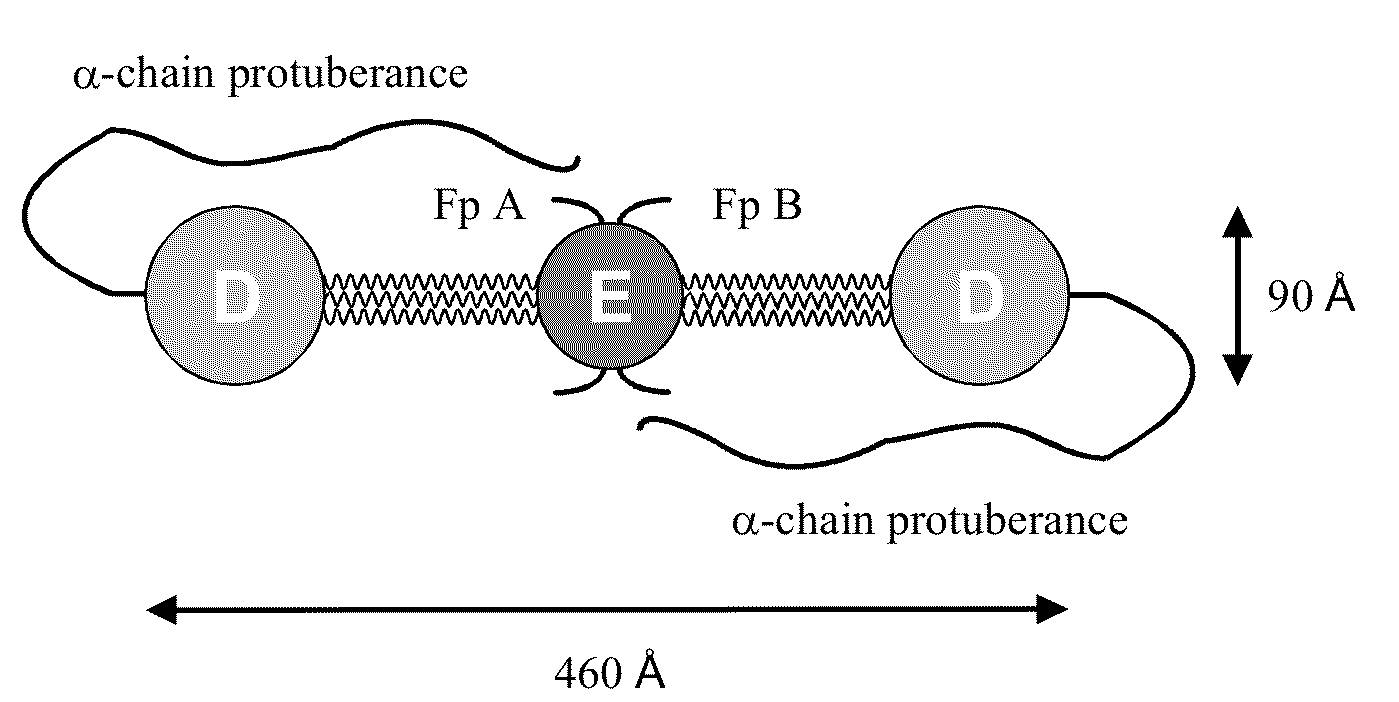
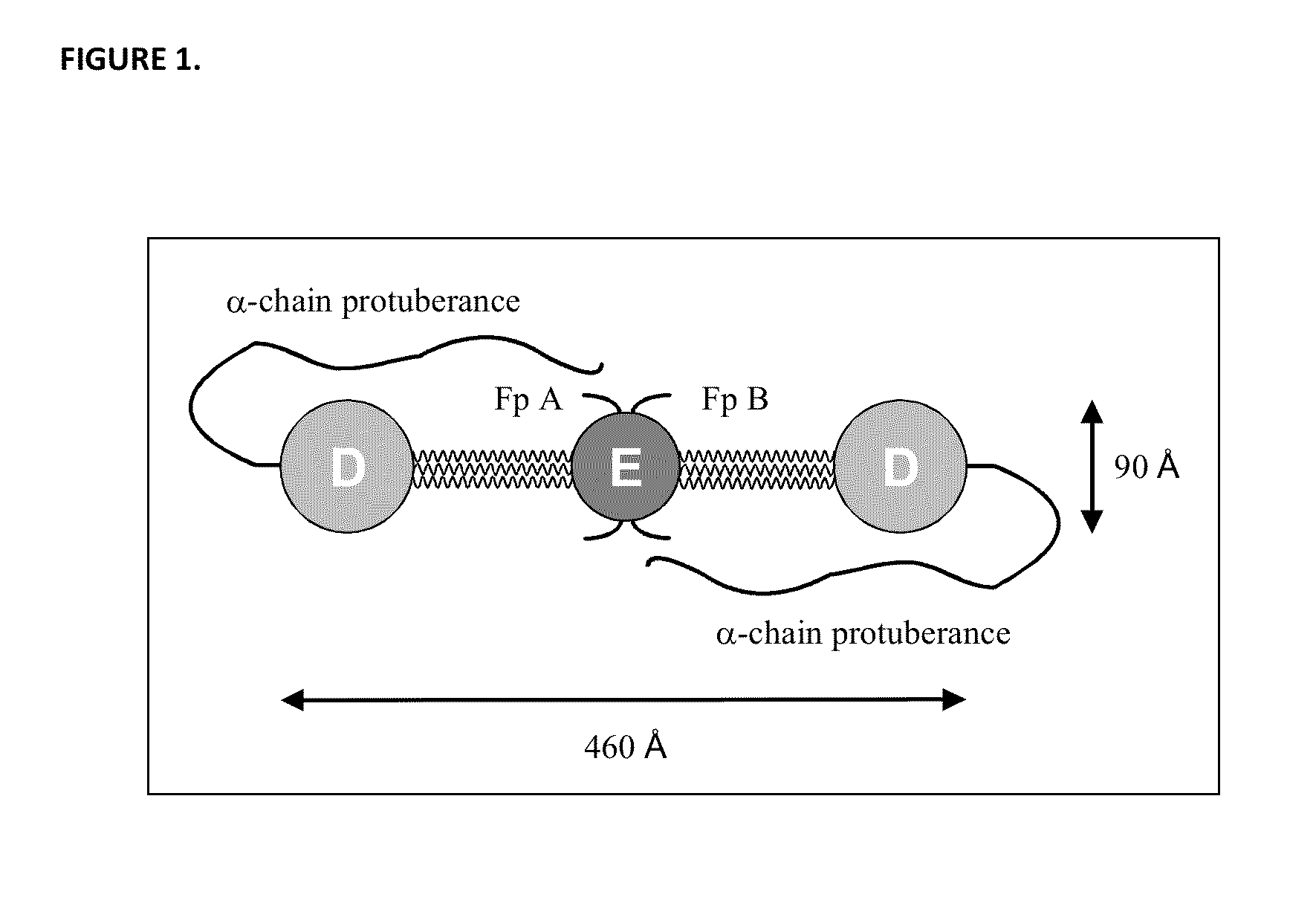
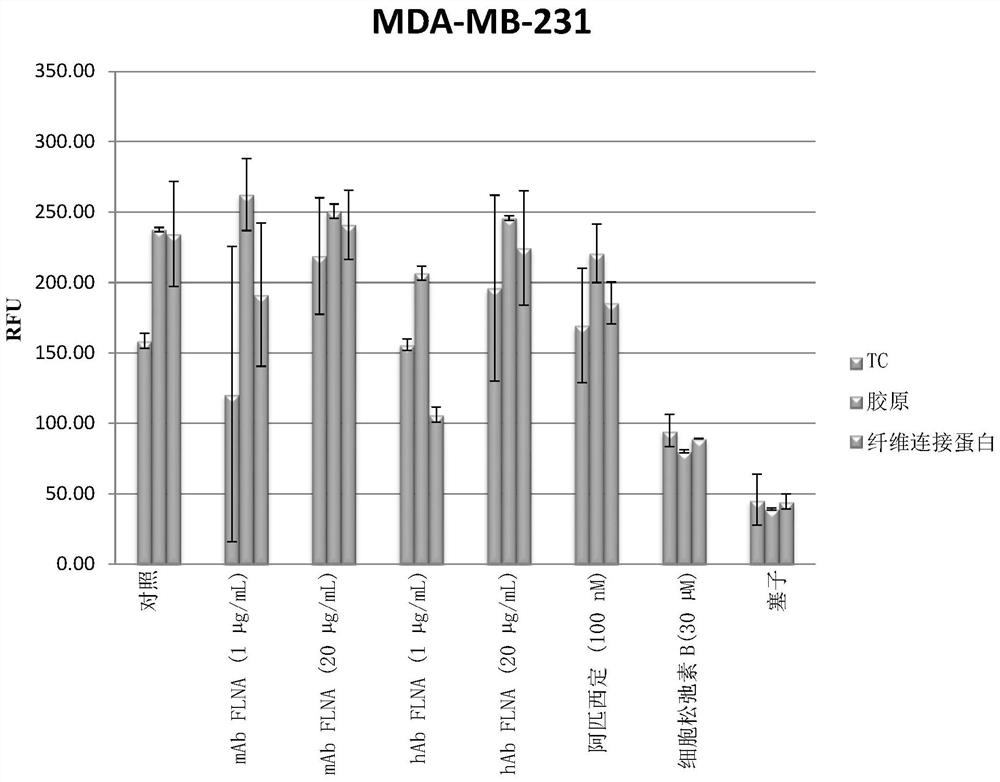
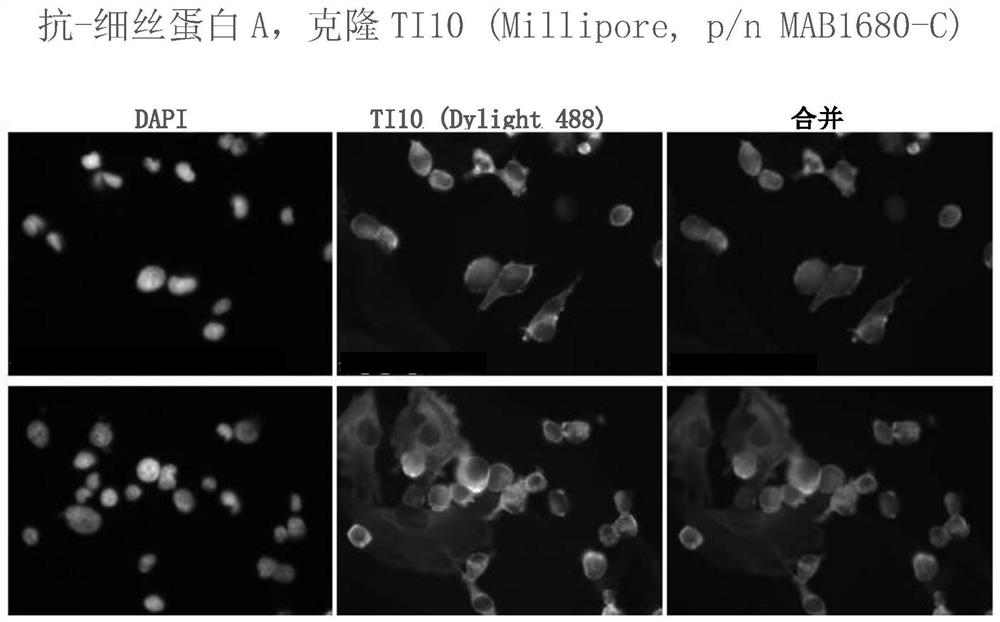
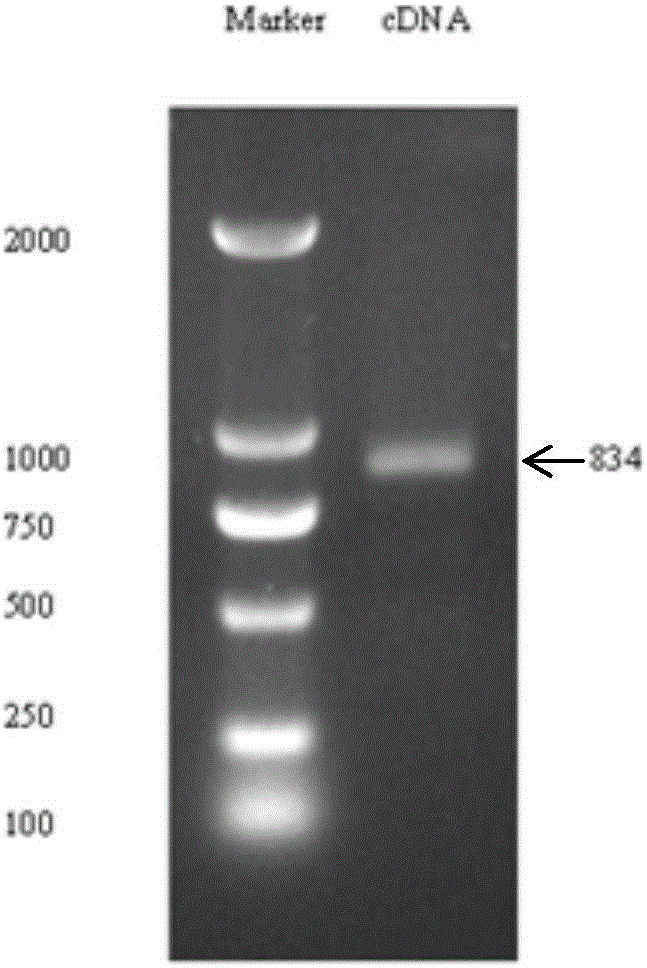
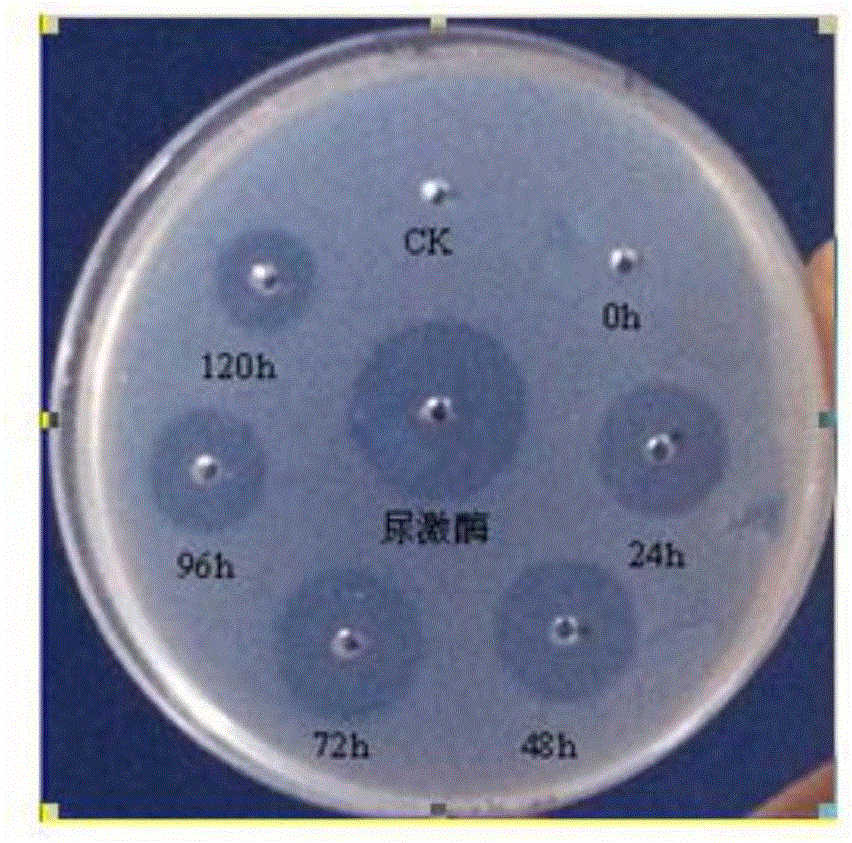
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Filamin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Synthetic phosphodiester oligonucleotides and therapeutical uses thereof
InactiveUS20110092576A1Organic active ingredientsGenetic material ingredientsDiseaseEthylene Homopolymers
A composition of phosphodiester oligonucleotides of various defined sizes has been created that mimics the effects of defibrotide. The composition essentially consists of mixtures of synthetic phosphodiester oligonucleotides comprising Nmers ranging from 40 mers to 65 mers. The phosphodiester oligonucleotides are preferably heteropolymers composed of either A, G, C, and T at each position but may also be homopolymers, i.e. the same base may be present at each position in the oligonucicotidc. These mixtures are effective in the treatment of cancer and other diseases.
Owner:GENTIUM SRL
Methods and compositions for diagnosing neoplastic disease
InactiveUS20080293162A1Blocking may occurReduce probabilityImmunoglobulins against animals/humansBiological material analysisAnalyteWilms' tumor
Methods and compositions for determining whether a subject at least has a neoplastic disease are provided. In practicing the subject methods, a sample from a subject is assayed for a soluble filamin analyte, such as a filamin A analyte, to determine whether the subject at least has the neoplastic disease. Also provided are kits, systems, and devices for practicing the subject methods.
Owner:LIFE TECH CORP
Use of markers including filamin a in the diagnosis and treatment of prostate cancer
ActiveUS20160258958A1Accurate and reliable detectionEfficient and accurate and rapid diagnosisMicrobiological testing/measurementBiological material analysisProstate cancerKeratin 7
Methods for diagnosing the presence of prostate cancer in a subject are provided, such methods including the detection of levels of variety of biomarkers diagnostic of prostate cancer, including filamin A alone, or in combination with one or more additional biomarkers of prostate cancer, including, PSA, keratin 4, keratin 7, keratin 8, keratin 15, keratin 18, keratin 19, tubulin-beta 3, filamin B, and LY9. Additionally, age can be used as a predictor variable. The invention also provides methods of treating prostate cancer which rely on diagnostic information obtained based on the detection of biomarkers of prostate cancer, including filamin A alone, or in combination with one or more additional biomarkers of prostate cancer, including, PSA, keratin 4, keratin 7, keratin 8, keratin 15, keratin 18, keratin 19, tubulin-beta 3, filamin B, LY9, and / or age. Compositions in the form of kits and panels of reagents for detecting the biomarkers of the invention are also provided.
Owner:BPGBIO INC
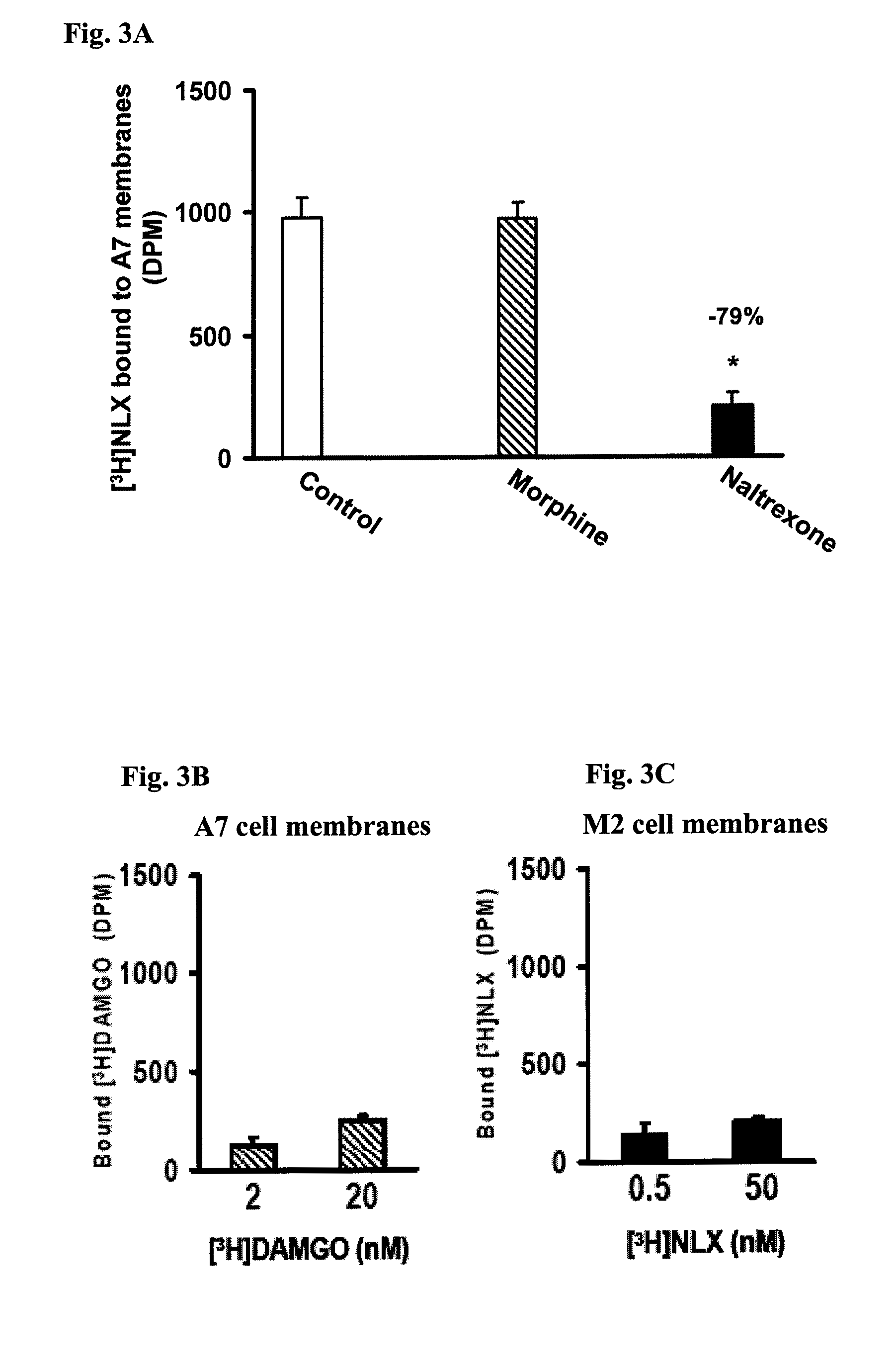
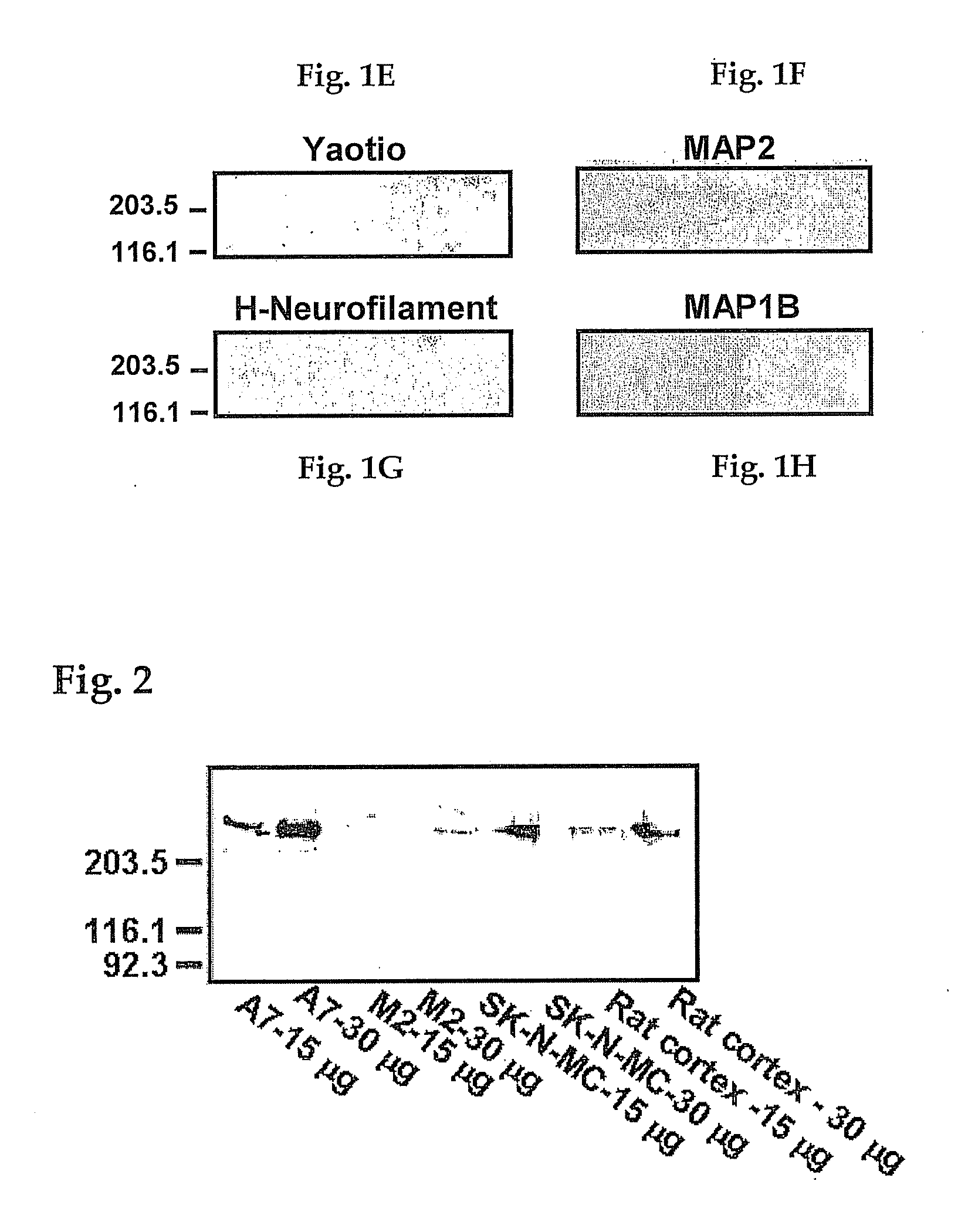
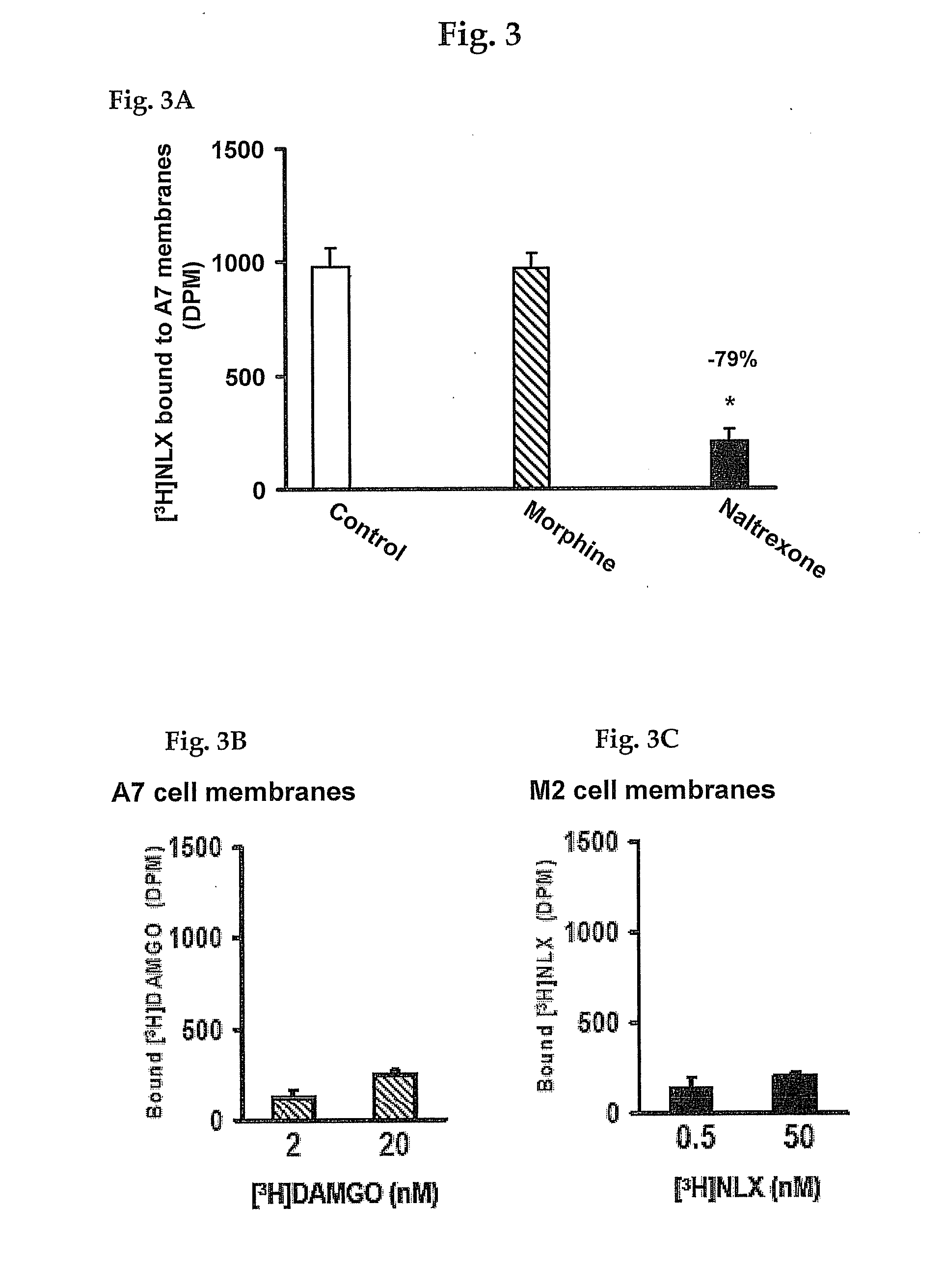
Analgesia with minimal tolerance and dependence by a mu opioid receptor agonist that also binds filamin a
ActiveUS20090191579A1Minimizing and preventing opioid toleranceMinimizing and preventing and dependencePeptide/protein ingredientsMicrobiological testing/measurementMu-Opioid Receptor AgonistsOpioid receptor
A composition and method are disclosed that utilize an isolated polypeptide or analog thereof to inhibit the interaction of a mu-opioid receptor with filamin A. A contemplated polypeptide has an amino acid residue sequence illustrated by the formula: W-[X1X2X3 . . . X43X44X45]nValAlaX48GlyLeu[X51X52X53 . . . X94X95X96]m-Y, wherein the various elements are defined elsewhere. A contemplated method can be used to select a VAKGL-binding compound.
Owner:PAIN THERAPEUTICS INC
Methods and compositions for diagnosing neoplastic disease
InactiveUS20060063214A1Decreasing risk of deathArresting its developmentImmunoglobulins against animals/humansBiological material analysisDiseaseAnalyte
Methods and compositions for determining whether a subject at least has a neoplastic disease are provided. In practicing the subject methods, a sample from a subject is assayed for a soluble filamin analyte, such as a filamin A analyte, to determine whether the subject at least has the neoplastic disease. Also provided are kits, systems, and devices for practicing the subject methods.
Owner:BIOSOURCE INT
Use of markers in the diagnosis and treatment of prostate cancer
InactiveUS20140038838A1Increased riskGreat predictive valuePeptide librariesMicrobiological testing/measurementProstate cancerKeratin 4
Owner:BERG
Methods and compositions for identifying a cellular immune response against prostate cancer
ActiveUS7901902B2Peptide/protein ingredientsMicrobiological testing/measurementProstate cancer cellB peptides
Filamin-B peptides, compositions comprising such peptides, and methods of using such peptides to assess an immune response against such peptides are described. An immune response against the peptides correlates with an immune response, in particular a cellular immune response, against prostate cancer cells which immune response is preferably associated with prophylaxis of prostate cancer, treatment of prostate cancer, and / or amelioration of at least one symptom associated with prostate cancer.
Owner:HEALTHTREE INC
Method of inhibiting tau phosphorylation
ActiveUS10017736B2Inhibition formationReduce impactNervous disorderOrganic chemistryPharmacophorePharmaceutical medicine
A method of inhibiting phosphorylation of the tau protein and / or a TLR4-mediated immune response is disclosed. The method contemplates administering to cells in recognized need thereof such as cells of the central nervous system an effective amount of a of a compound or a pharmaceutically acceptable salt thereof that binds to a pentapeptide of filamin A (FLNA) of SEQ ID NO: 1, and contains at least four of the six pharmacophores of FIGS. 35-40.
Owner:PAIN THERAPEUTICS INC
Method for expressing Reteplase in Escherichia coli by using dual plasmid system
InactiveCN102250933AEfficient soluble expressionSimple production processBacteriaHydrolasesFibrin plateAmpicillin
The invention relates to a method for expressing Reteplase (rPA) in Escherichia coli by using a dual plasmid system. The method comprises the following steps of: performing polymerase chain reaction (PCR) amplification to obtain gene segment of the rPA, and cloning the gene segment into a vector pET22b to construct a recombinant plasmid pET22b-rPA; co-transforming the pET22b-rPA and pET40b into Escherichia coli BL21 (DE3) by using different resistances of the pET22b-rPA and the pET40b, and screening engineering bacteria under ampicillin (Amp) and kanamycin (Kan) double resistance selection pressure; and performing inducible expression on the engineering bacteria by using isopropyl-beta-D-1-thiogalactopyranoside (IPTG) to obtain the soluble rPA target protein. Through detection by a fibrin plate method, the rPA protein obtained in the method is not needed to be subjected to renaturation and has obvious thrombolytic activity.
Owner:TIANJIN UNIV OF SCI & TECH
Methods and compositions for typing molecular subgroups of medulloblastoma
ActiveUS20140155400A1Quick and accurate identificationOrganic active ingredientsDisease diagnosisPositive controlBeta-catenin
Immunohistochemical methods and compositions for the typing of molecular subgroups of medulloblastomas are provided. The methods comprise determining a protein expression profile for a sample obtained from a medulloblastoma by detecting expression of GAB 1, filamin A, or at least two biomarker proteins selected from the group consisting of β-catenin, YAP1, GAB1, and filamin A, and typing the medulloblastoma as a WNT pathway tumor, a SHH pathway tumor, or a non-WNT / non-SHH tumor based on this protein expression profile. Kits for typing a medulloblastoma according to these three molecular subgroups are provided. The kits comprise at least two antibodies, wherein each of said antibodies specifically binds to a distinct biomarker protein selected from the group consisting of β-catenin, YAP1, GAB1, and filamin A, and can optionally comprise one or more of instructions for use, reagents for detecting antibody binding to one or more of said biomarker proteins, and one or more positive control samples.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Composition for preventing or treating keloid or hypertrophic scar
PendingCN112839664AOrganic active ingredientsCompound screeningEukaryotic initiation factorThioredoxin
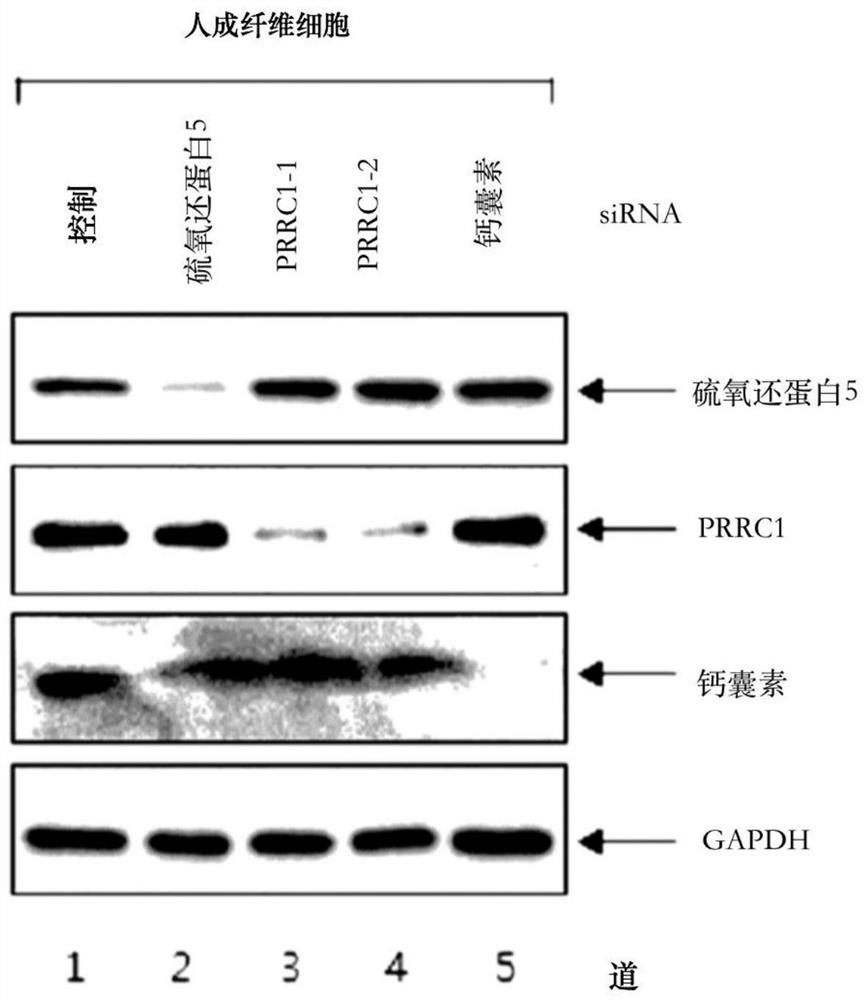
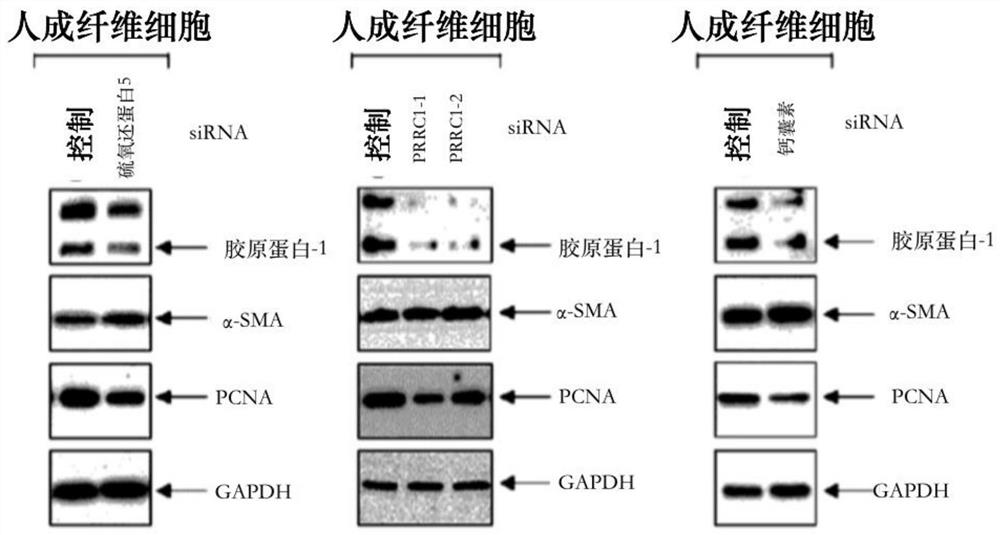
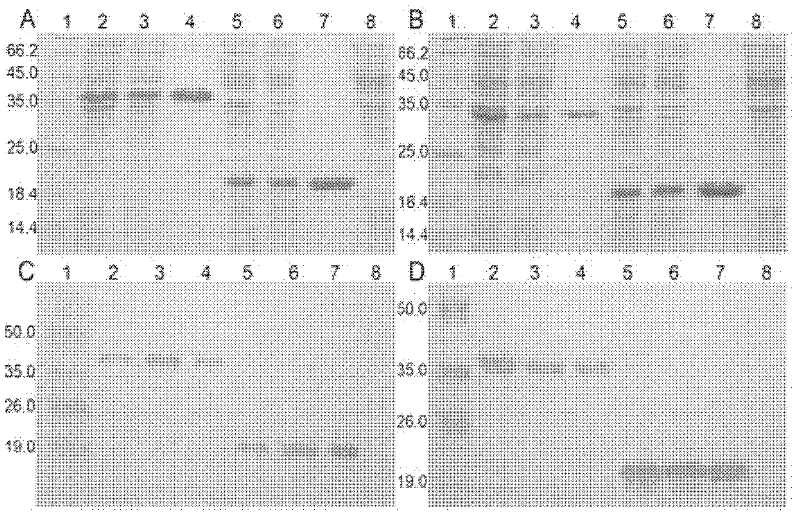
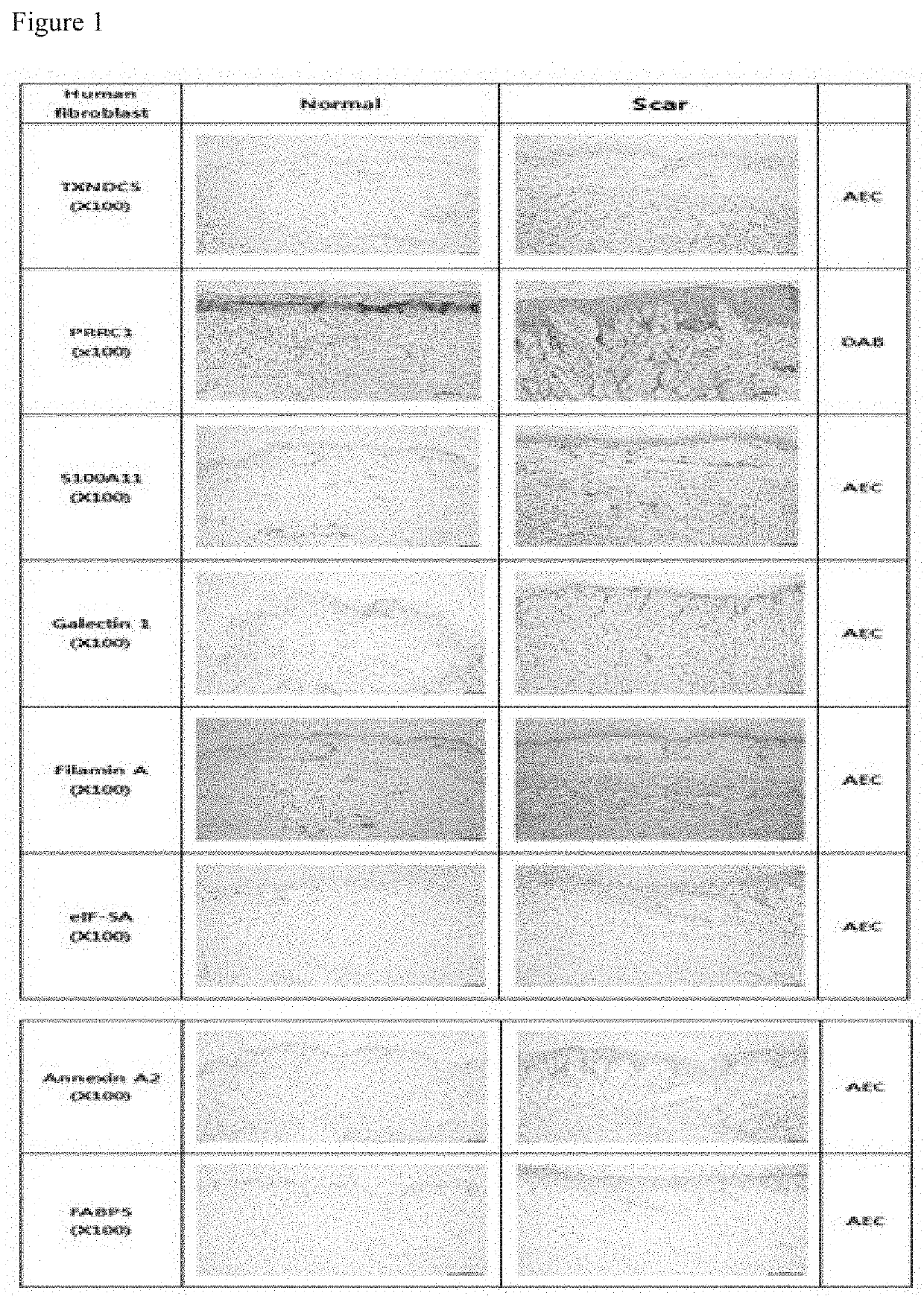
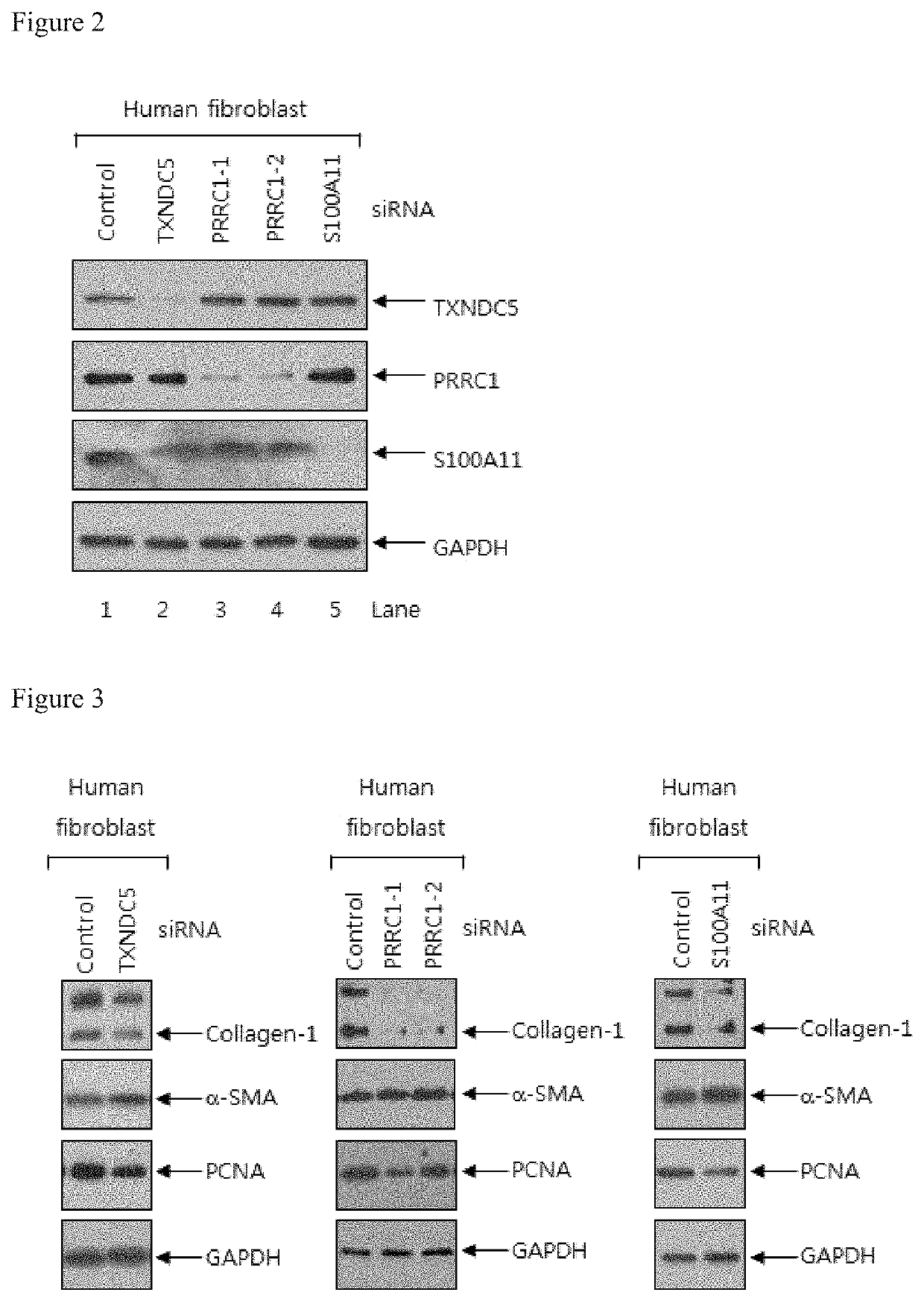
The present invention relates to a pharmaceutical composition for preventing or treating a hypertrophic scar. The present inventors acknowledged that expression inhibition of thioredoxin 5, PRRC1, S100A11, galectin 1, filamin A, eukaryotic initiation factor-5A, annexin A2 and fatty acid binding proteins 5 can be a new target for relieving and treating a hypertrophic scar. According to the present invention, the treatability of a hypertrophic scar is confirmed by producing thioredoxin 5-, PRRC1-, S100A11-, galectin 1-, filamin A-, eukaryotic initiation factor-5A-, annexin A2- and FABP5-specific siRNAs. Therefore, the knockdown of the proteins or genes coding same induces cell death in a hypertrophic scar and reduces collagen expression and thus can be highly useful for scar treatment.
Owner:TEGO SCI
Recombinant protein specifically bound with fibrin and application thereof
ActiveCN102399293AAvoid low target specificityEnhances the effect of therapeutic vascularizationFungiBacteriaProtein targetAmino acid
The invention discloses a recombinant protein specifically bound with fibrin and the application thereof. The protein is a fusion protein which is obtained by fusing the following polypeptides a) or b) or c) or d) as fusion partners to an amino terminal of a target protein: a) a polypeptide prepared from an amino acid sequence as shown in the 22-107 positions from an amino terminal of sequence 2 in the sequence table; b) the polypeptide prepared from the amino acid sequence as shown in the 22-109 positions from the amino terminal of sequence 4 in sequence table; c) a polypeptide which is derived from a) and is prepared after replacement and / or deletion and / or addition of one or more amino acids from the amino acid sequence restricted by a) and can be specifically bound with fibrin; d) a polypeptide which is derived from b) and is prepared after replacement and / or deletion and / or addition of one or more amino acids from the amino acid sequence restricted by b) and can be specifically bound with fibrin. The recombinant protein specifically bound with fibrin of the invention can be used for wound restoration and active tissue induction regeneration.
Owner:INST OF GENETICS & DEVELOPMENTAL BIOLOGY CHINESE ACAD OF SCI
Composition for preventing or treating keloids or hypertrophic scars
InactiveUS20210388352A1Accurate diagnosisInhibits expression activityOrganic active ingredientsCompound screeningKeloidGalectin-1
The present invention relates to a pharmaceutical composition for preventing or treating hypertrophic scars. The present inventors have found that the inhibition of expression of TXNDC5, PRRC1, S100A11, Galectin 1, Filamin A, eIF-5A, Annexin A2, and FABP5 can be a new target for improving and treating hypertrophic scars. In the present invention, TXNDC5-, PRRC1-, S100A11-, Galectin 1-, Filamin A-, eIF-5A-, Annexin A2-, and FABP5-specific siRNAs were constructed to determine the probability of treating the hypertrophic scars. As a result, the knockdown of the protein or a gene encoding the protein induces apoptosis in the hypertrophic scars and reduces collagen expression, which can be very useful in treating wounds.
Owner:TEGO SCI
Use of markers in the diagnosis and treatment of prostate cancer
ActiveUS20160178632A1Increased riskIncrease valueMicrobiological testing/measurementDisease diagnosisProstate cancerKeratin 4
Owner:BPGBIO INC
Analgesia with minimal tolerance and dependence by a mu opioid receptor agonist that also binds filamin a
ActiveUS20120237951A1Avoid problemsAvoid interactionPeptide/protein ingredientsAnalogue computers for chemical processesMu-Opioid Receptor AgonistsChemistry
A composition and method are disclosed that utilize an isolated polypeptide or analog thereof to inhibit the interaction of a mu-opioid receptor with filamin A. A contemplated polypeptide has an amino acid residue sequence illustrated by the formula: W—[X1X2X3 . . . X43X44X45]nValAlaX48GlyLeu[X51X52X53 . . . X94X95X96]m—Y wherein the various elements are defined elsewhere. A contemplated method can be used to select a VAKGL-binding compound.
Owner:PAIN THERAPEUTICS INC
Use of markers including filamin A in the diagnosis and treatment of prostate cancer
ActiveUS10539566B2Accurate and reliable detectionEfficient and accurate and rapid diagnosisMicrobiological testing/measurementBiological material analysisProstate cancerBiologic marker
Owner:BPGBIO INC
Fibrinogen Assay
ActiveUS20130130282A1Inhibition of thrombin activityElectrolysis componentsVolume/mass flow measurementFiberMonoclonal antibody
The present invention is directed to a method of detecting intact fibrinogen, comprising the steps of: a) providing a sample containing at least some fibrinogen optionally converted at least in part to fibrin, and optionally containing thrombin; b) solubilizing the sample in a solubilizing solution that inhibits thrombin activity; c) after optional SDS-PAGE transferring / applying a portion of said sample to a protein-binding membrane; d) reacting the fibrinogen with a primary monoclonal antibody capable of binding to fibrinopeptide A moiety; and e) detecting the quantity of intact fibrinogen in the sample by quantifying the amount of the bound primary monoclonal antibody.
Owner:ETHICON INC
D-dimer, troponin, and nt-probnp for pulmonary embolism
InactiveUS20110129936A1Analysis using chemical indicatorsLaboratory glasswaresFibrinogen degradation productIntensive care medicine
The present invention relates to a method of diagnosing acute pulmonary embolism (PE) in a subject including a) determining the amount of fibrin-fibrinogen degradation products, in particular D-dimer in a sample of the subject; b) determining the amount of a natriuretic peptide in a sample of the subject; c) determining the amount of a cardiac troponin in a sample of the subject; and d) comparing the amounts determined in steps a) to c) to reference amounts, thereby establishing the diagnosis. Included is also a method of deciding on a therapy of a subject diagnosed with PE and a method of monitoring the therapy.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
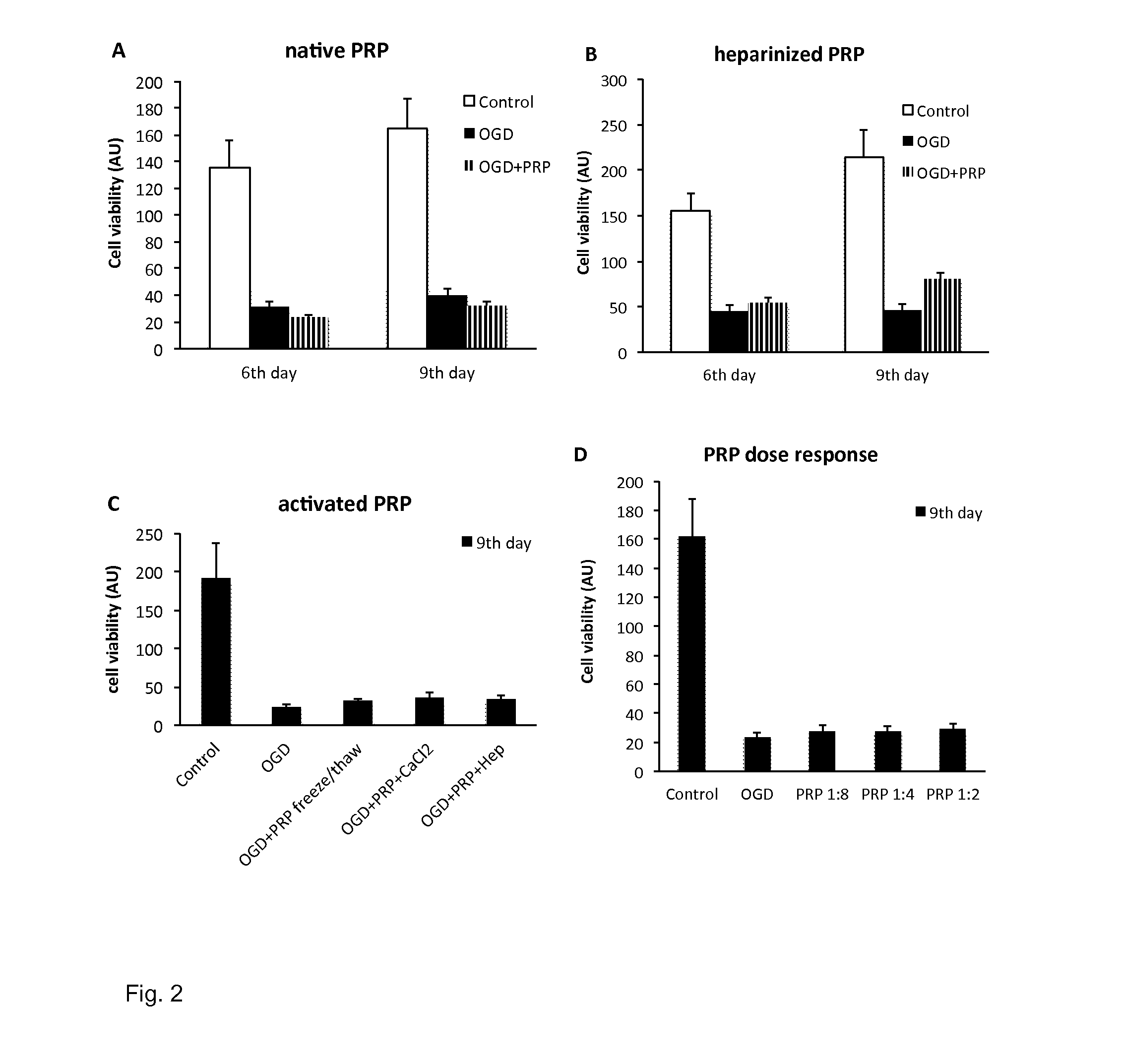
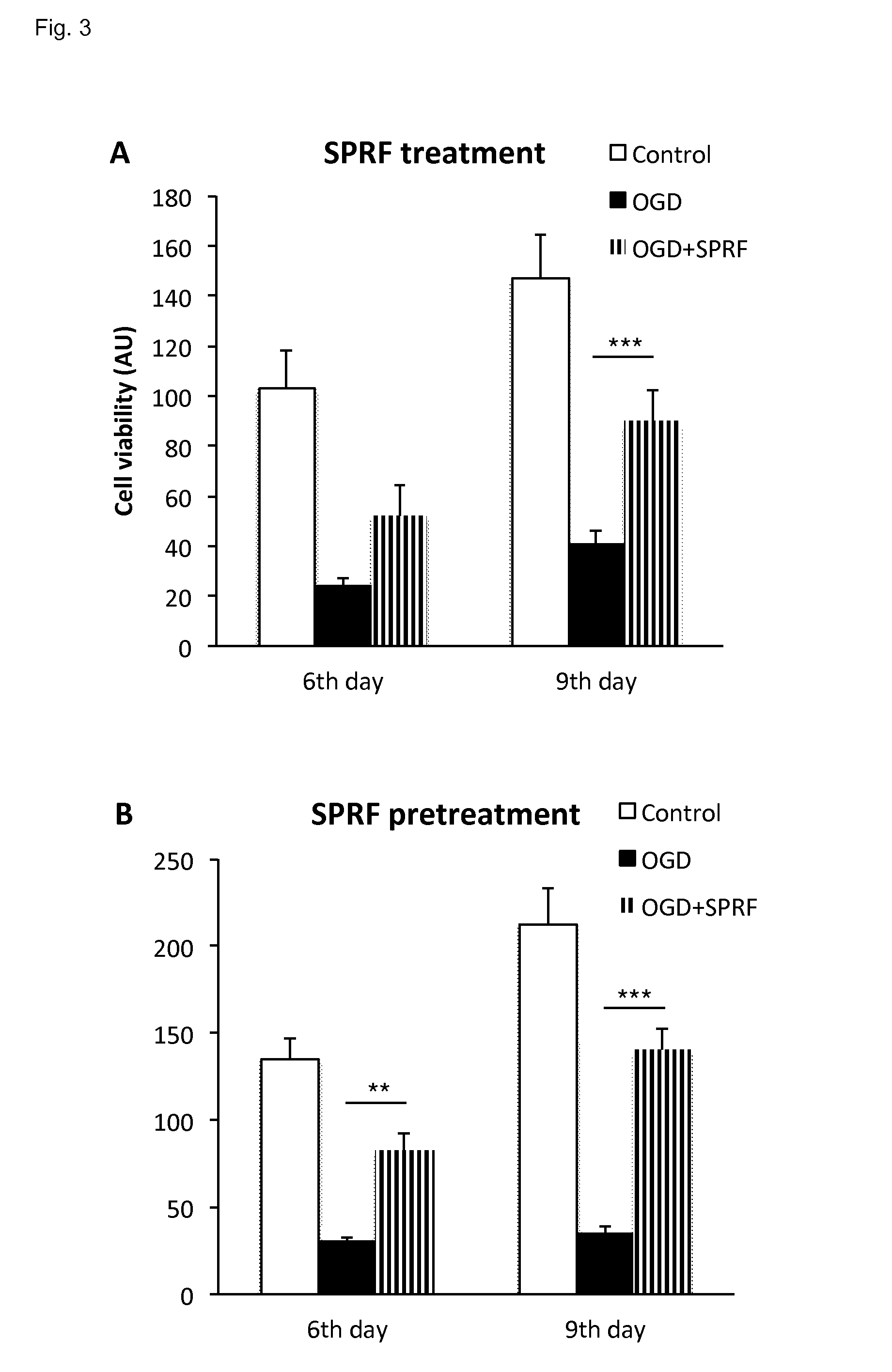
Serum fraction of platelet-rich fibrin
ActiveUS9480716B2Efficient activationMaintaining the integrity of platelet structuresBiocideInanimate material medical ingredientsAnticoagulantPlatelet release
The invention provides for a method of preparing an isolated serum fraction of platelet rich fibrin (PRF), comprising the steps ofa. providing platelet rich plasma (PRP) without the addition of an anticoagulant;b. clotting the PRP to obtain a coagel of PRF; andc. separating the coagel to isolate the serum fraction which comprises an activated platelet releasate;and further provides for the isolated serum fraction obtained by such method, and its medical use.
Owner:LACERTA TECH
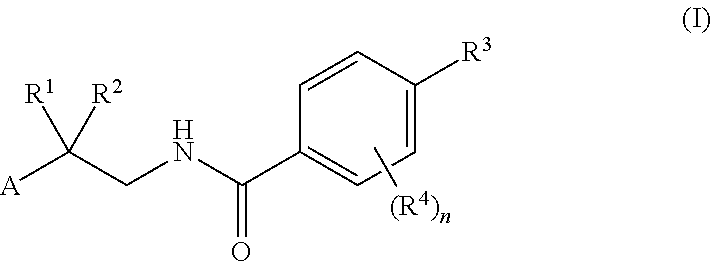
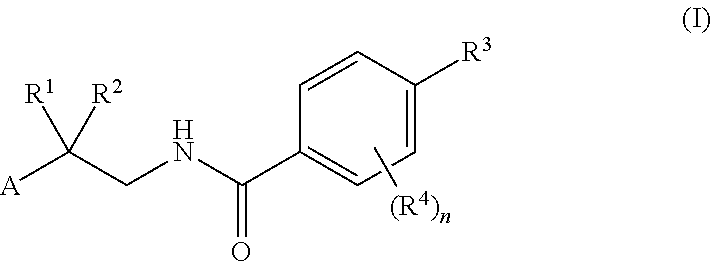
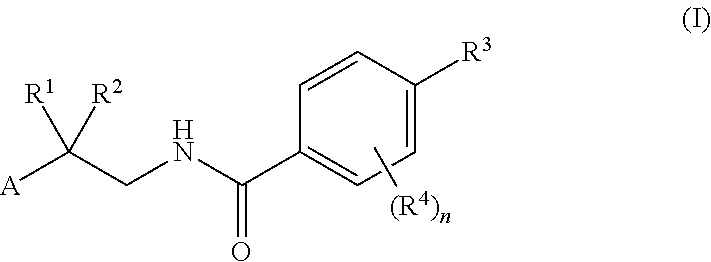
Drp1-FILAMIN COMPLEX FORMATION INHIBITORS
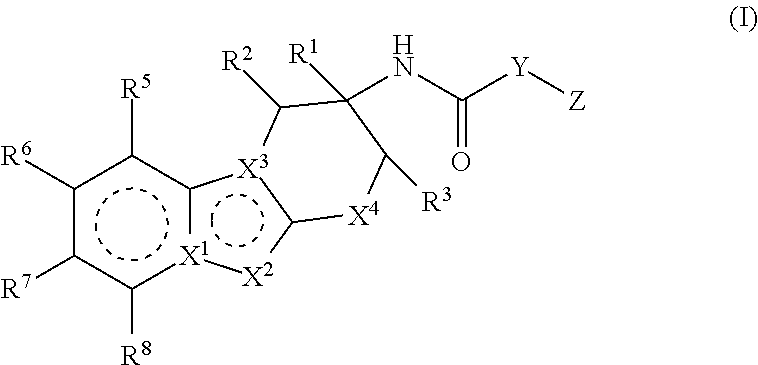
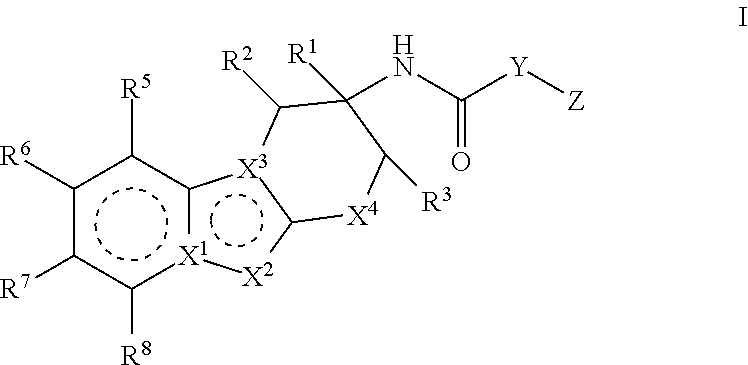
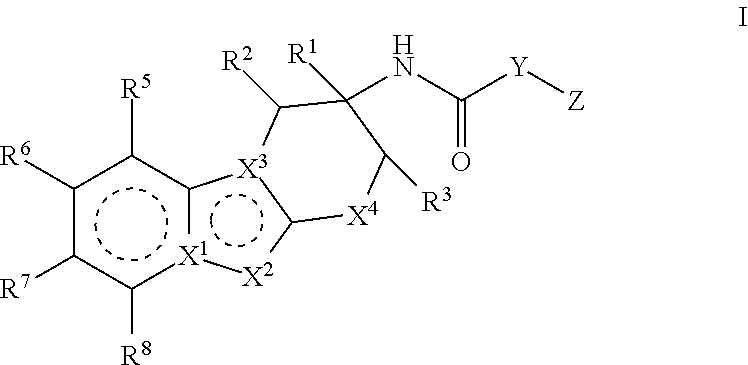
Compounds represented by formula (I) belowand pharmacologically acceptable salts thereof and solvates of them; pharmaceutical compositions and dynamin-related protein 1 (Drp1)-filamin complex formation inhibitors containing them; and uses of the compounds, salts, solvates, pharmaceutical compositions, and (Drp1)-filamin complex formation inhibitors for use as a prophylactic or therapeutic agent.
Owner:RDISCOVERY LLC
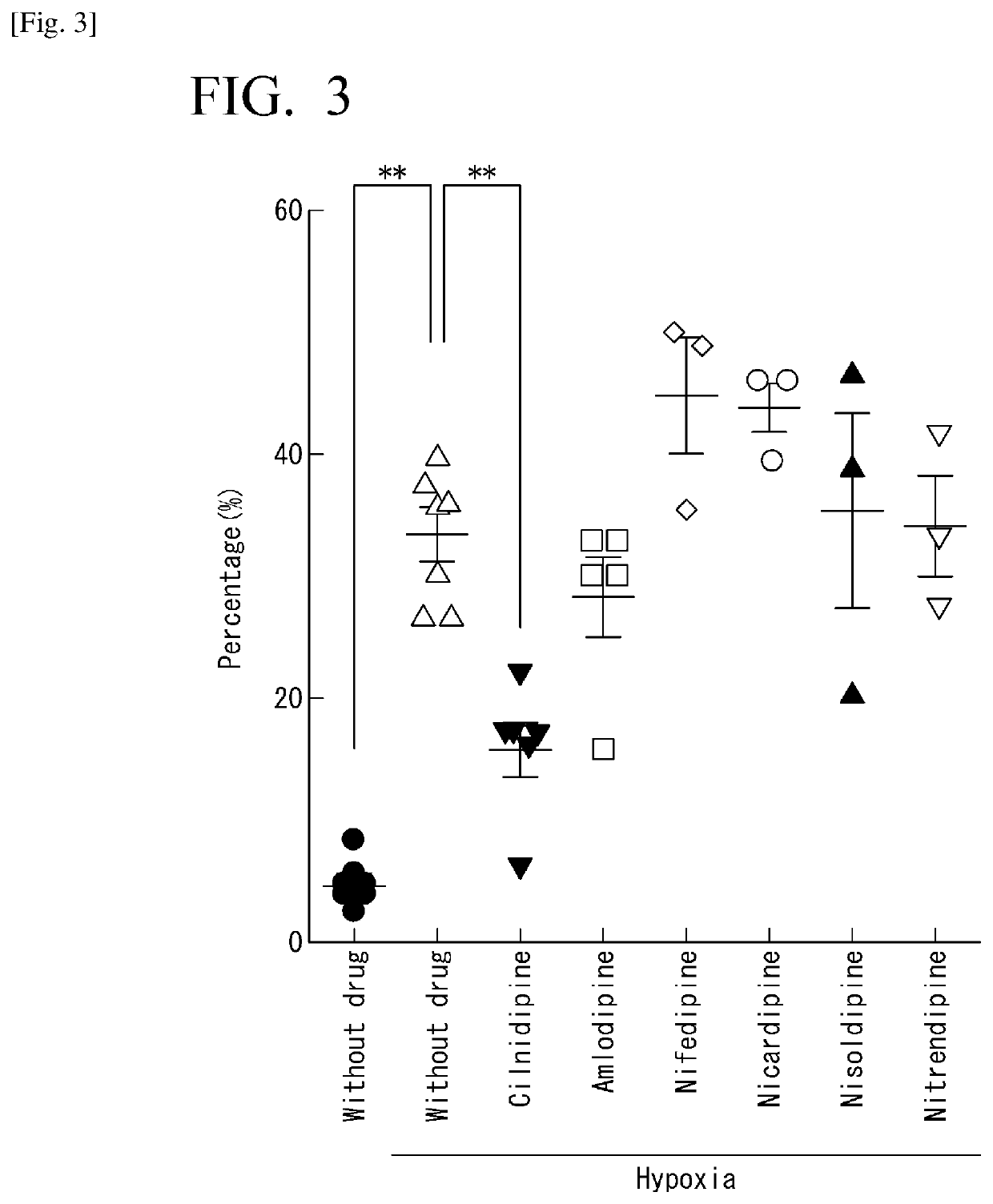
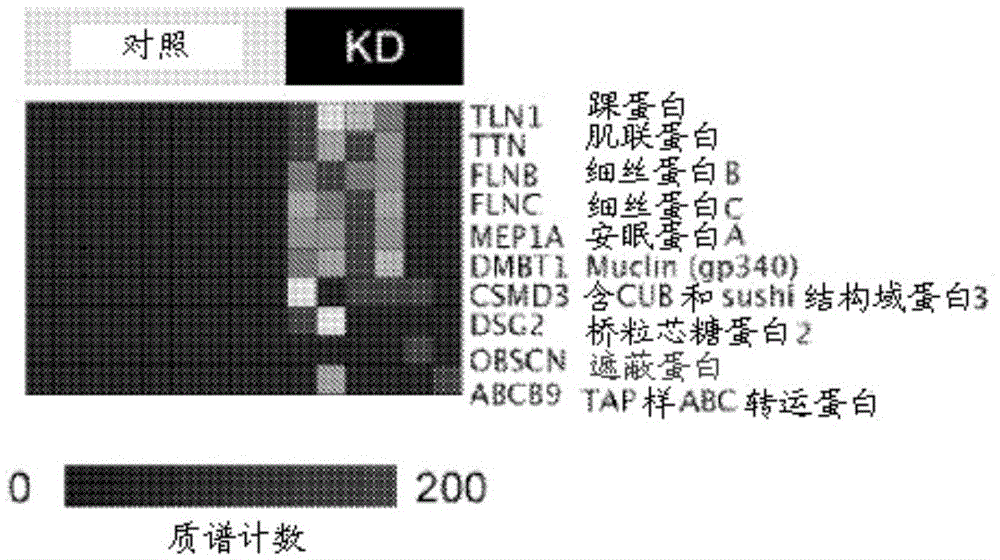
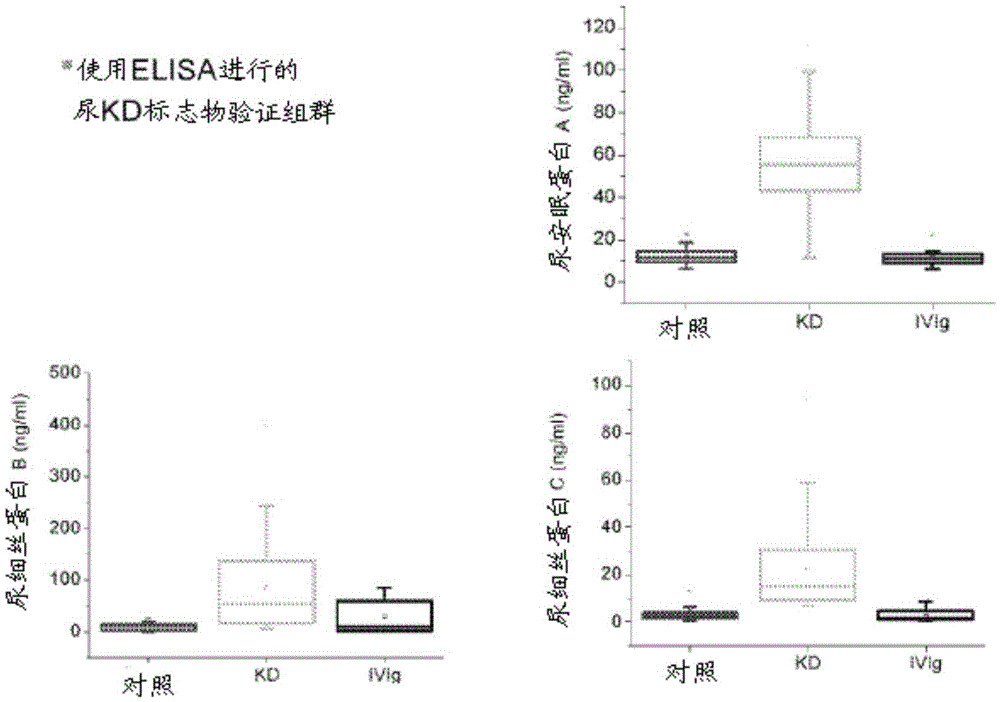
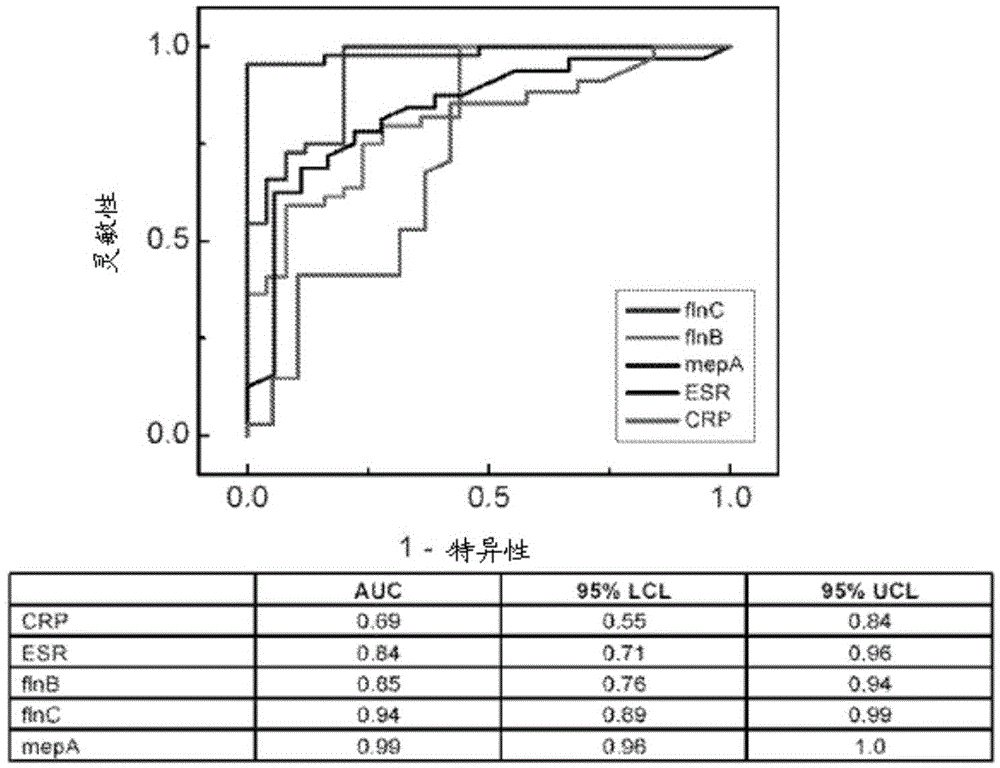
Diagnostic markers and therapeutic targets for Kawasaki disease
The present invention provides compositions and methods for the diagnosis and treatment of Kawasaki disease. More specifically, the proteome of KD patients is enriched in meprin A, filamin B, and filamin C, which serve as biomarkers (and potential therapeutic targets) for KD. Accordingly, detection of these biomarkers using the compositions and methods provided herein can inform the delivery of therapy to a subject.
Owner:CHILDRENS MEDICAL CENT CORP
Synthetic peptide materials for joint reconstruction, repair and cushioning
In joint reconstruction, repair and cushioning applications, a synthetic polypeptide material is useful that contains cross-linked polypeptides that are modeled on human elastin or other fibrous proteins. The polypeptides comprise at least three consecutive beta-sheet / beta-turn structures and at least one amino acid residue that participates in cross-linking.
Owner:ELASTIN SPECIALTIES +1
Antibodies directed to filamin-a and therapeutic uses thereof
PendingUS20220025025A1Stably and transiently expressedInhibit expressionBiological material analysisRadioactive preparation carriersHuman cancerAntiendomysial antibodies
The disclosure teaches antibodies that are useful, inter alia, in methods for detecting and treating human cancer. In a particular aspect, the disclosure teaches novel antibodies that are useful for detecting and treating human breast cancer. In some embodiments, the disclosure teaches novel antibodies that bind to filamin A. In some embodiments, the antibodies are intrabodies.
Owner:IBEX BIOSCI LLC
Antibodies directed to filamin-a and therapeutic uses thereof
ActiveCN113164633ALow immunogenicityHybrid immunoglobulinsBiological material analysisHuman cancerAntiendomysial antibodies
The disclosure teaches antibodies that are useful, inter alia, in methods for detecting and treating human cancer. In a particular aspect, the disclosure teaches novel antibodies that are useful for detecting and treating human breast cancer. In some embodiments, the disclosure teaches novel antibodies that bind to filamin A. In some embodiments, the antibodies are intrabodies.
Owner:IBEX BIOSCI LLC
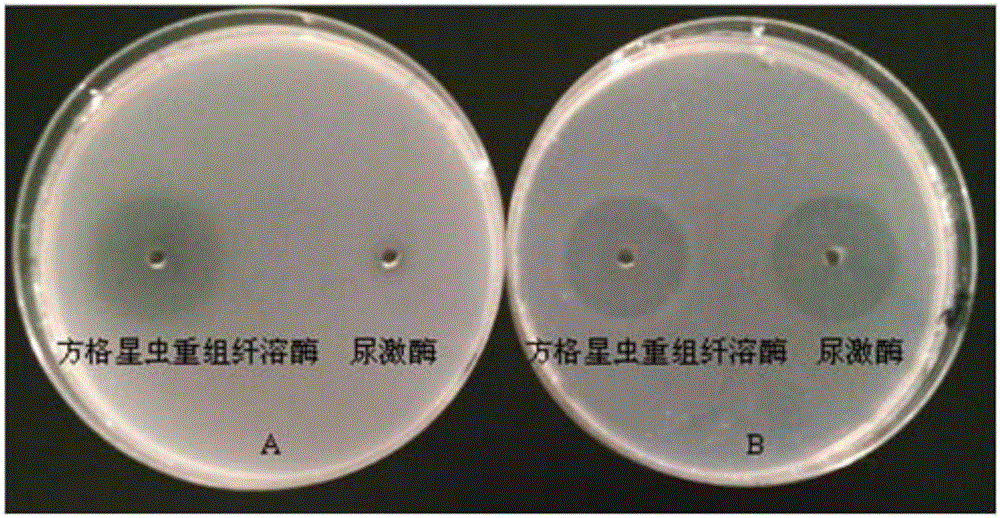
A sipunculus nudus plasmin cDNA gene, a recombined plasmin and applications of the recombined plasmin
The invention discloses a sipunculus nudus plasmin cDNA gene, a recombined plasmin and applications of the recombined plasmin and belongs to the technical field of genetic engineering. The sipunculus nudus plasmin cDNA gene sequence and the amino acid sequence thereof are shown as SEQ ID NO:1 and SEQ ID NO:2. The cDNA sequence encodes 834 nucleotides, namely 277 amino acids. Through a pGAPZaA vector, the sipunculus nudus plasmin cDNA gene is transferred into a pichia pastoris GS115 strain to perform secretory expression. The recombined plasmin can directly dissolve fibrin in thrombi, and can be used for preparing thrombolytic medicines to treat thrombotic diseases.
Owner:HAIKOU EXPERIMENTAL STATION CHINESE ACAD OF TROPICAL AGRI SCI
Factor IXa Inhibitors
The present invention provides a compound of Formula I, and pharmaceutical compositions comprising one or more said compounds, and methods for using said compounds for treating or preventing unstable angina, refractory angina, myocardial infarction, transient ischemic attacks, atrial fibrillation, thrombotic stroke, embolic stroke, deep vein thrombosis, disseminated intravascular coagulation, ocular build up of fibrin, and reocclusion or restenosis of recanalized vessels. The compounds are selective Factor IXa inhibitors.
Owner:MERCK SHARP & DOHME LLC +1
Factor IXa inhibitors
The present invention provides a compound of Formula I and pharmaceutical compositions comprising one or more said compounds, and methods for using said compounds for treating or preventing unstable angina, refractory angina, myocardial infarction, transient ischemic attacks, atrial fibrillation, thrombotic stroke, embolic stroke, deep vein thrombosis, disseminated intravascular coagulation, ocular build up of fibrin, and reocclusion or restenosis of recanalized vessels. The compounds are selective Factor IXa inhibitors.
Owner:MERCK SHARP & DOHME LLC
Filamin b binding proteins and uses thereof
InactiveUS20200024335A1Improve the level ofAbnormal stateImmunoglobulins against animals/humansAnimals/human peptidesAntiendomysial antibodiesProstate cancer
The present invention encompasses filamin B (FLNB) binding proteins. Specifically, the invention relates to antibodies to FLNB. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. Methods of making and methods of using the antibodies of the invention in methods of diagnosis, monitoring and prognosis or prostate cancer are also provided.
Owner:BERG
Methods and compositions for typing molecular subgroups of medulloblastoma
ActiveUS9005907B2Quick and accurate identificationDisease diagnosisBiological testingPositive controlBeta-catenin
Immunohistochemical methods and compositions for the typing of molecular subgroups of medulloblastomas are provided. The methods comprise determining a protein expression profile for a sample obtained from a medulloblastoma by detecting expression of GAB1, filamin A, or at least two biomarker proteins selected from the group consisting of β-catenin, YAP1, GAB1, and filamin A, and typing the medulloblastoma as a WNT pathway tumor, a SHH pathway tumor, or a non-WNT / non-SHH tumor based on this protein expression profile. Kits for typing a medulloblastoma according to these three molecular subgroups are provided. The kits comprise at least two antibodies, wherein each of said antibodies specifically binds to a distinct biomarker protein selected from the group consisting of β-catenin, YAP1, GAB1, and filamin A, and can optionally comprise one or more of instructions for use, reagents for detecting antibody binding to one or more of said biomarker proteins, and one or more positive control samples.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
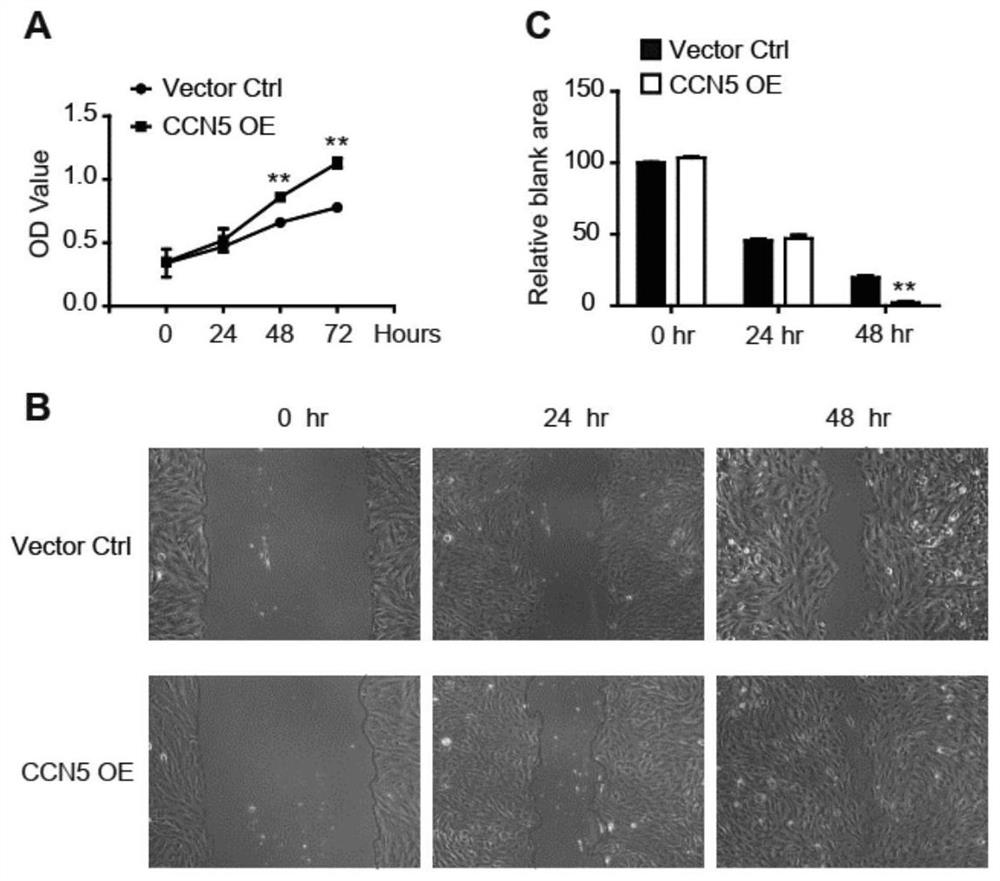
Stromal cell protein CCN5 composition and application thereof
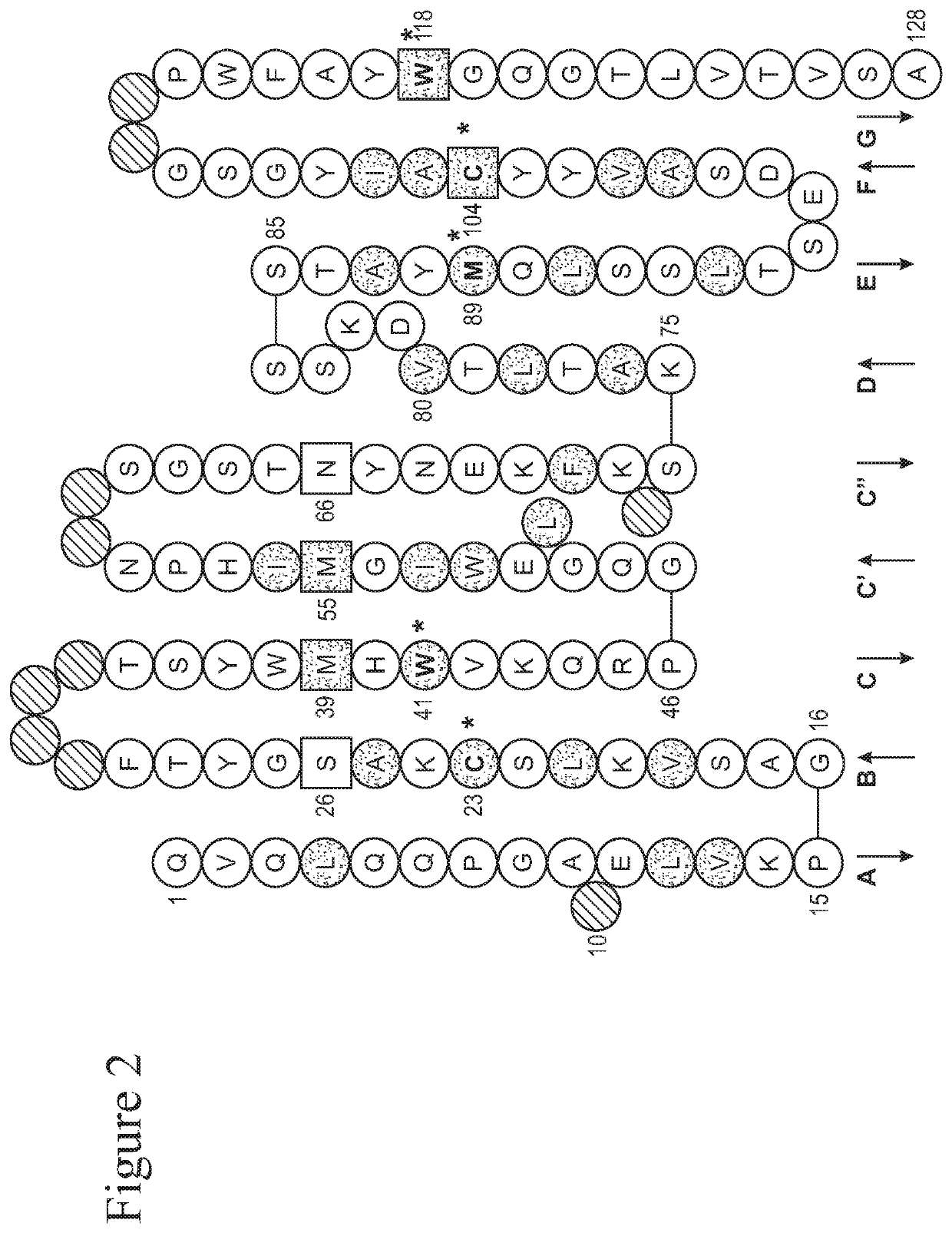
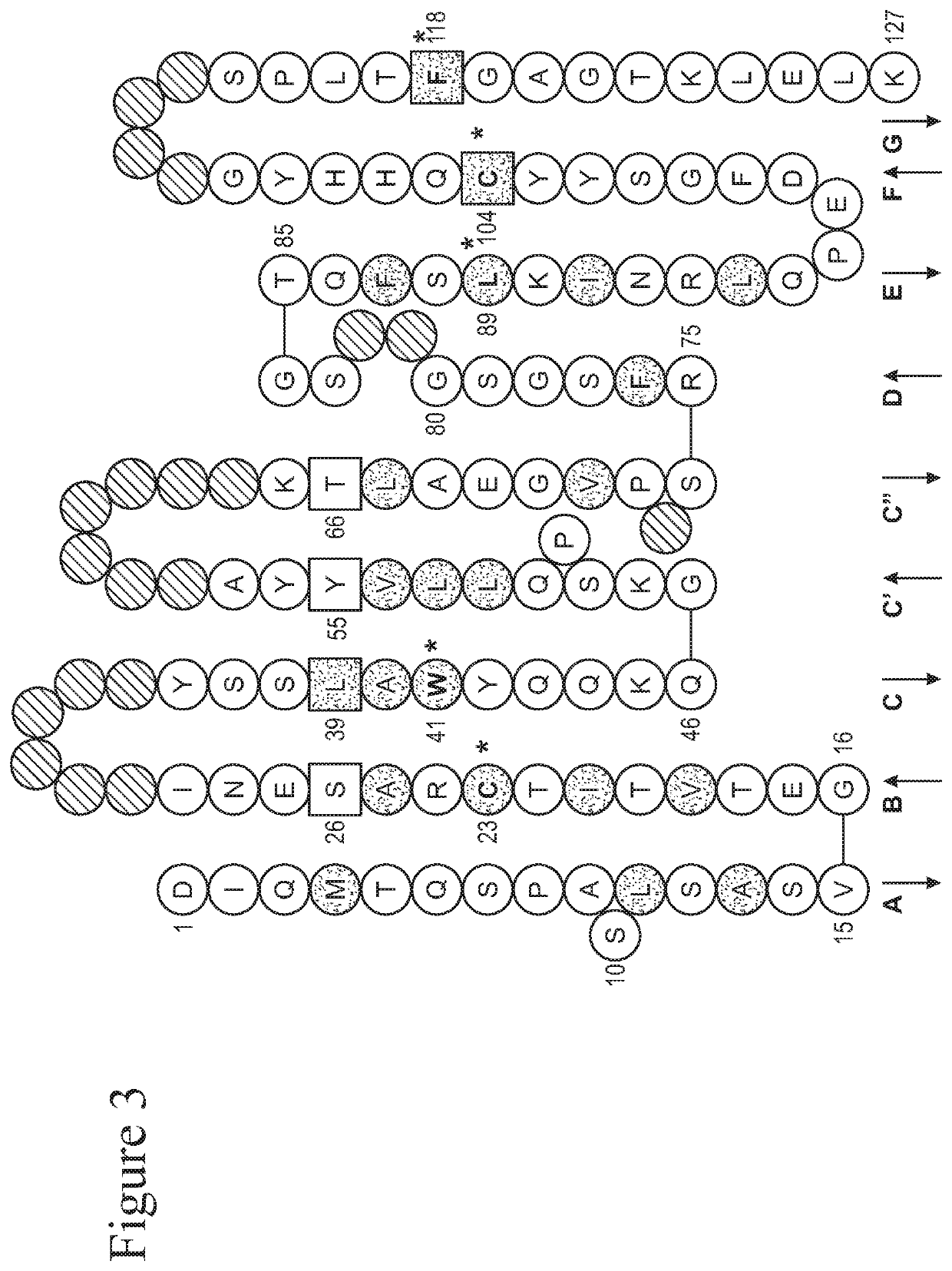
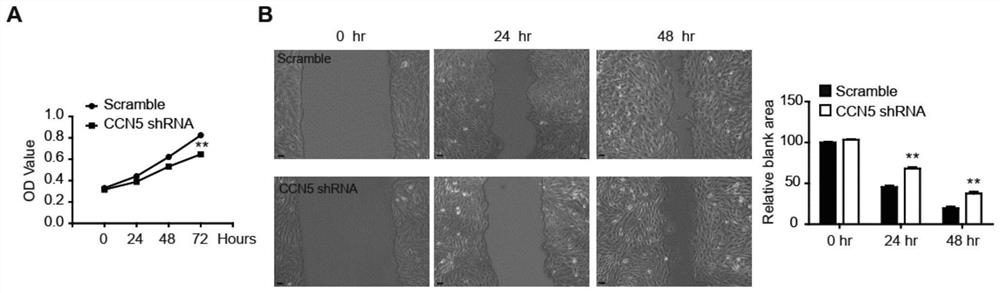
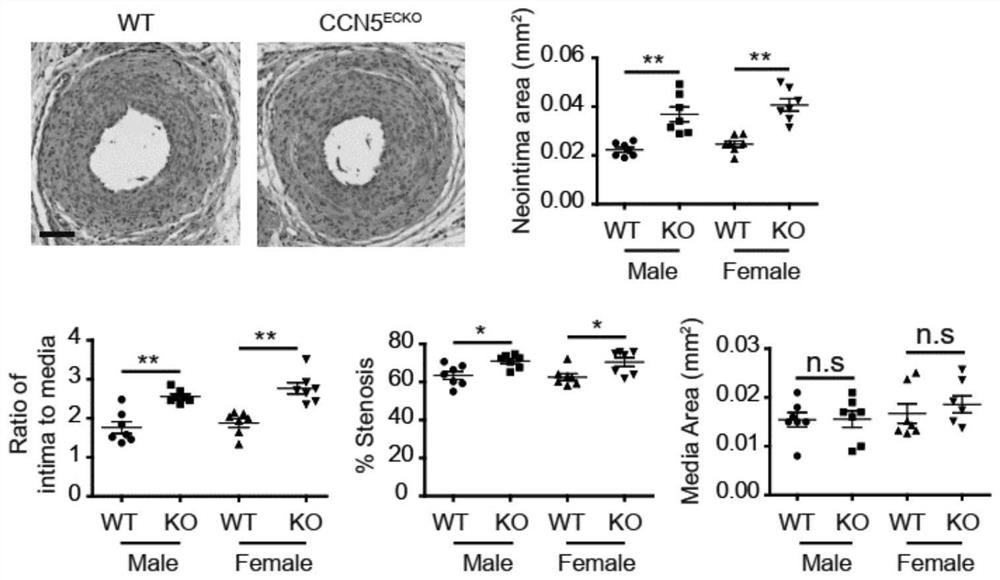
PendingCN112190709APromote proliferationPromote migrationOrganic active ingredientsAntipyreticPlatelet inhibitionIntimal hyperplasia
A cell communication network factor 5 (CCN5, alias WISP2, CTGFL, CT58) is a member of a stromal cell protein CCN family, and is expressed in various cells and other tissue cells constituting a blood vessel. It is proved in the patent that vascular endothelium and smooth muscle cells CCN5 play different roles in injured angiogenesis intimal hyperplasia, endothelial cells CCN5 can promote injured endothelial cell repair to inhibit intimal hyperplasia by combining an integrin signal path and other mechanisms, and can also inhibit neogenesis intimal hyperplasia by regulating the function of smoothmuscle cells through paracrine; the smooth muscle CCN5 can regulate and control the functions of vascular smooth muscle cells through Filamin A or Thymosin beta 4 or other mechanisms to inhibit neogenesis intimal hyperplasia; and in addition, the recombinant CCN5 protein can also inhibit platelet aggregation. The invention provides application of a CCN5 gene, CCN5 protein, CCN5 active polypeptideor an accelerant thereof, and the CCN5 gene, CCN5 protein, CCN5 active polypeptide or the accelerant thereof are used for preparing a composition or a preparation, and the composition or the preparation is used for treating cardiovascular diseases such as vascular restenosis after coronary heart disease interventional therapy.
Owner:SHANGHAI EAST HOSPITAL EAST HOSPITAL TONGJI UNIV SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com