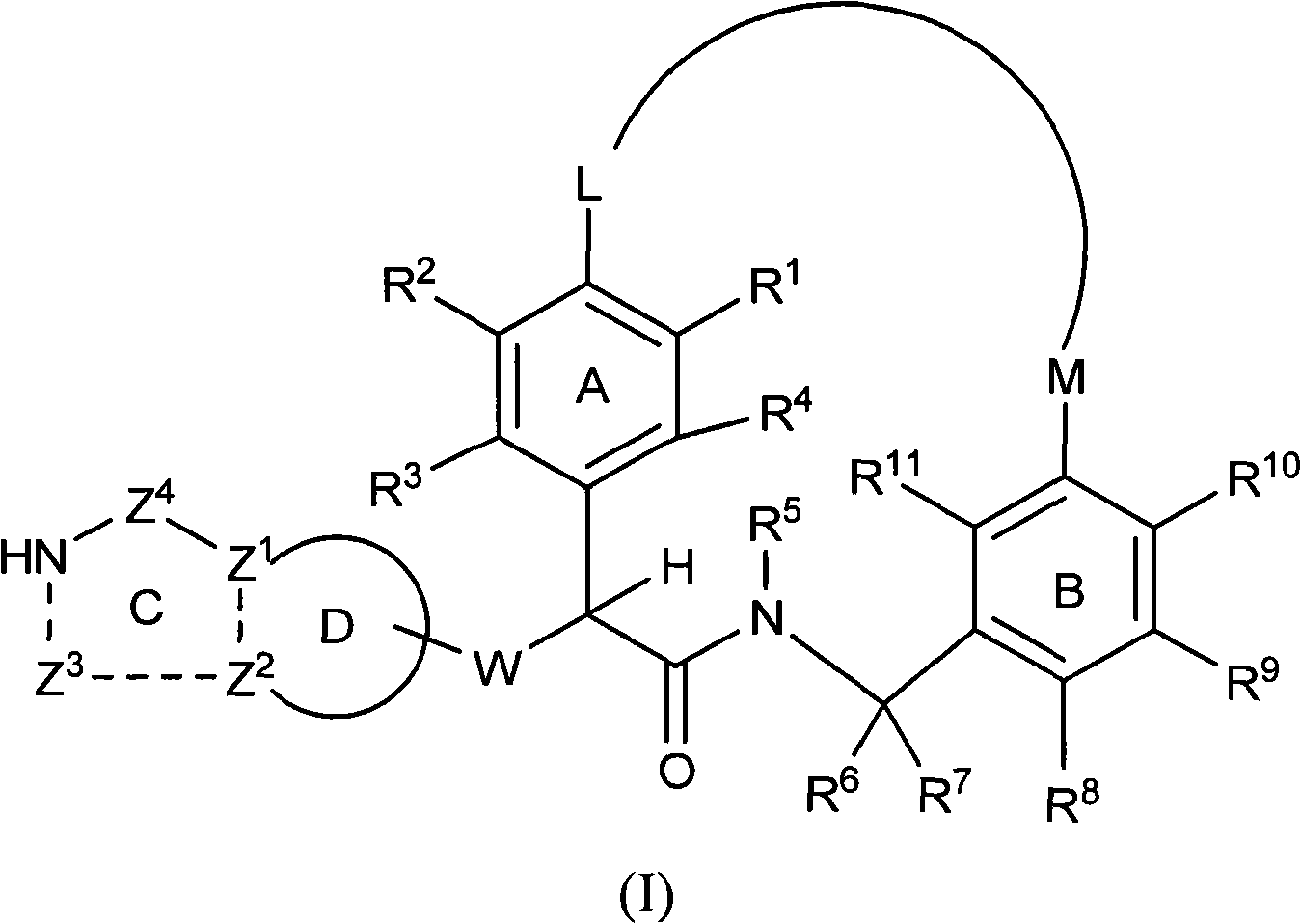
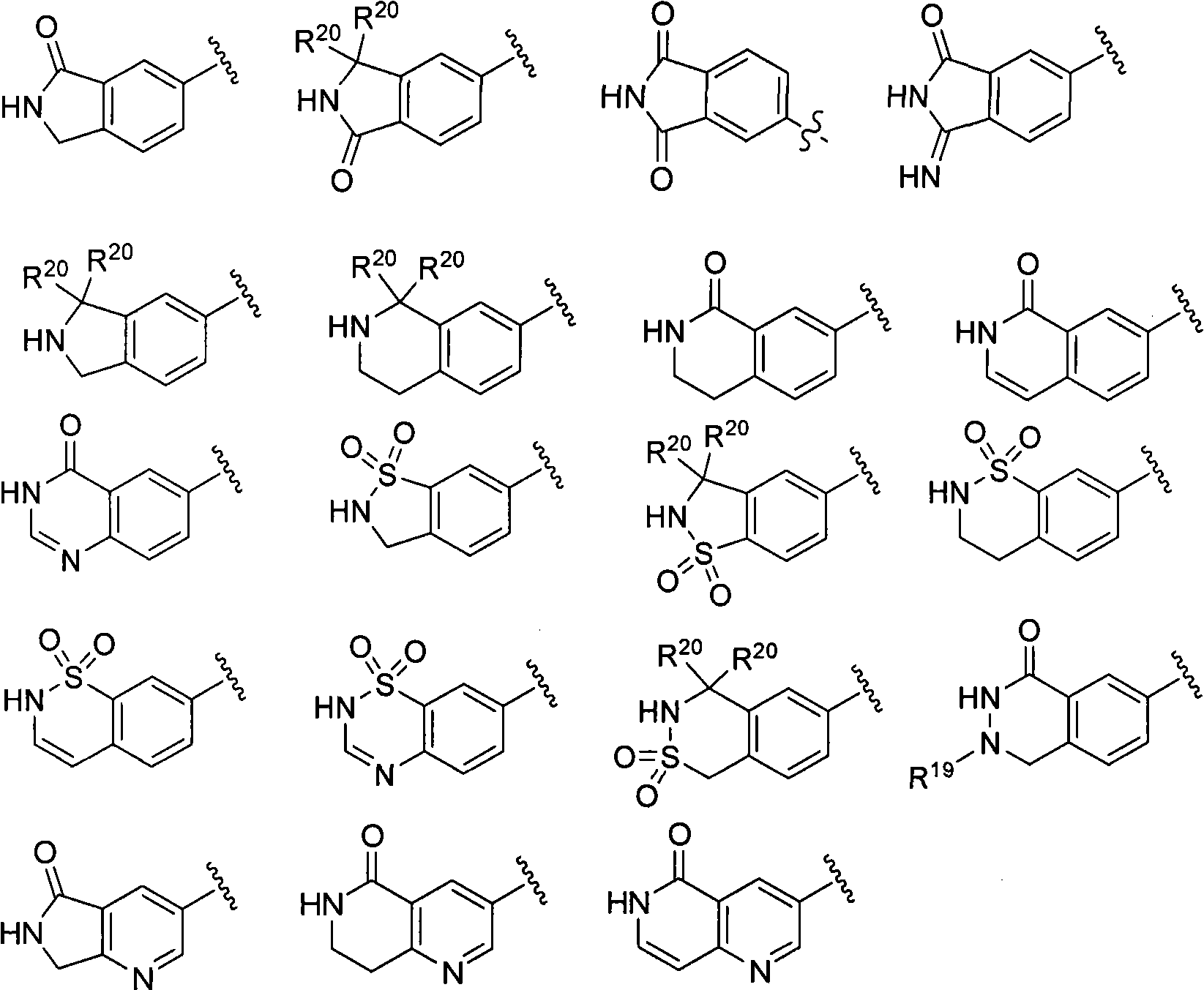
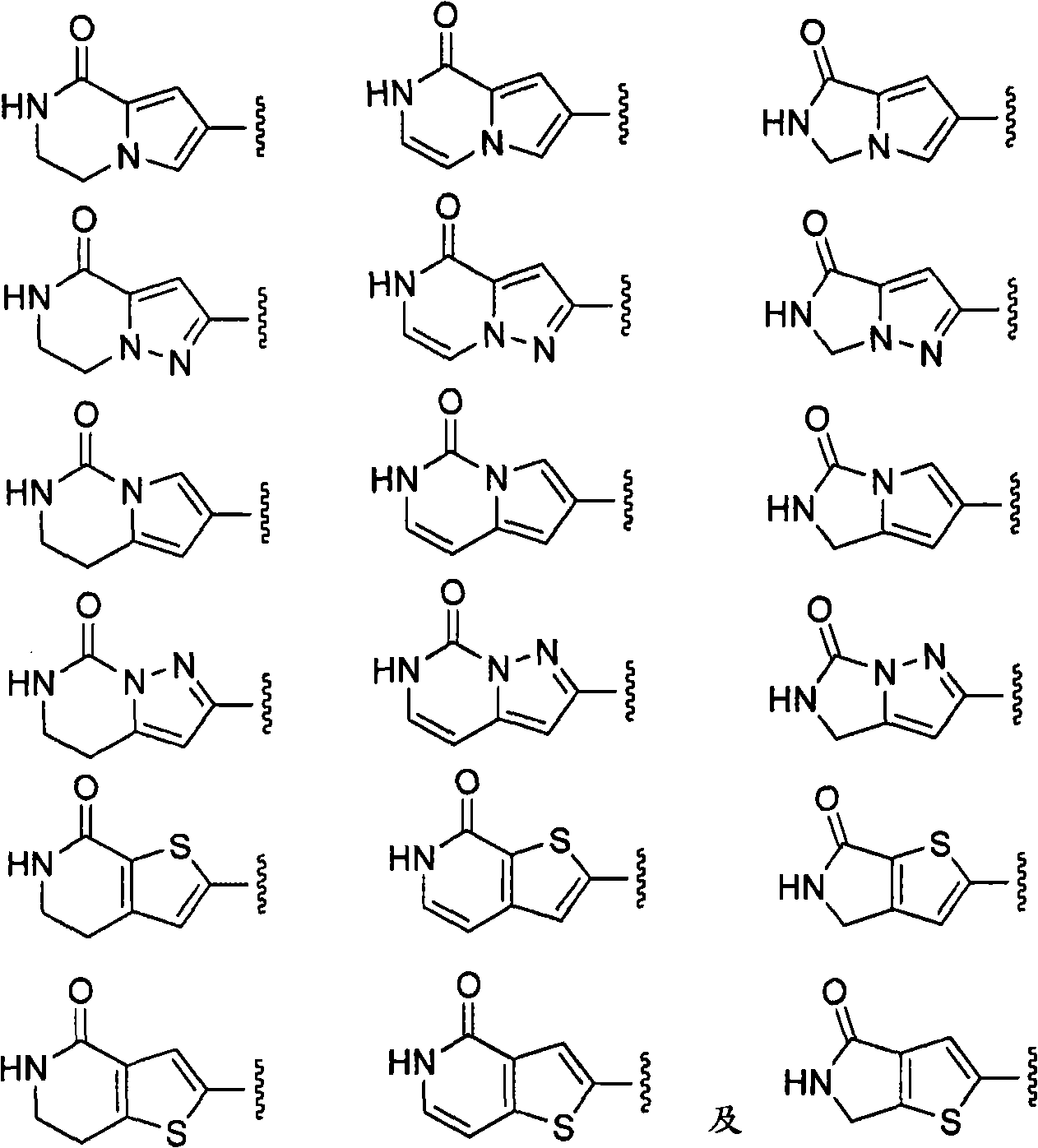
Macrocyclic factor VIIa inhibitors useful as anticoagulants
A technology of atoms and medicinal salts, applied in the field of macrocyclic coagulation factor VIIa inhibitors that can be used as anticoagulant drugs, can solve the problems of limited use, slow therapeutic effect, narrow therapeutic index, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0274] The preparation of prodrugs is well known in the art and described for example in Medicinal Chemistry: Principles and Practice, ed. F.D. King, The Royal Society of Chemistry, Cambridge, UK, 1994 .
[0275]The application also provides isotopically labeled compounds of the present invention, that is, wherein one or more of the atoms is replaced by the isotope of the atom (for example, C is replaced by 13 C or 14 C instead; and isotopes of hydrogen include tritium and deuterium). Such compounds have a variety of potential uses, for example, as standards or reagents in determining the ability of a potential pharmaceutical compound to bind a target protein or receptor, or for in vivo or in vitro testing of compounds of the invention that bind to biological receptors. for imaging.
[0276] The compounds of the invention are preferably isolated and purified after their preparation to obtain a composition ("substantially pure") comprising equal to or greater than 98%, prefer...
Embodiment 1
[0631] Example 1: (R)-7-ethanesulfonyl-2-(3-oxo-2,3-dihydro-1H-isoindol-5-ylamino)-4,11-diaza-tri ring [14.2.2.1 6,10 ] Hexa-1(19), 6, 8, 10(21), 16(20), 17-hexaene-3, 12-dione
[0632]
[0633] Add ethanethiol (2.8 mL, 38 mmol) to 2-fluoro-5-nitrobenzonitrile (5.00 g, 30.1 mmol) and triethylamine (9.3 mL, 67 mmol) in DMF (100 mL ) in the solution. The reaction mixture was stirred for 1 hour, then poured into water (500 mL). The formed precipitate was isolated by filtration, dissolved in DCM, washed with water and brine, dried (MgSO 4 ), and concentrated under reduced pressure. The residue (6.14 g) was dissolved in DCM (100 mL), cooled to 0 °C and treated with mCPBA (16.0 g, 71 mmol) in one portion. The reaction mixture was stirred at room temperature overnight, then extracted with sodium bicarbonate solution (sat.), sodium bisulfite solution (10%) and brine. Dry the organic layer (MgSO 4 ), and concentrated under reduced pressure to afford 1A (5.6 g, 80%) as a pale ...
Embodiment 2
[0644] Example 2: (R)-7-ethanesulfonyl-2-(1-oxo-1,2,3,4-tetrahydro-isoquinolin-7-ylamino)-4,11-diazepine - tricyclic [14.2.2.1 6,10 ] Hexa-1(19), 6, 8, 10(21), 16(20), 17-hexaene-3, 12-dione
[0645]
[0646] Using a procedure similar to that used to prepare 1E, 1D (500 mg, 0.99 mmol) and intermediate 1 (161 mg, 0.99 mmol) were reacted with glyoxylic acid monohydrate (91 mg, 0.99 mmol) to afford 2A (643 mg, 0.812 mmol, 96% yield) as a yellow foam. MS(ESI)m / z679.5(M+H) + .
[0647] Example 2
[0648] Using a procedure similar to that used to prepare Example 1, 2A (640 mg, 0.943 mmol) was deprotected with TFA and cyclized with BOP to afford the racemic macrocycle (150 mg, 28.4% yield), which It is a yellow solid. The racemate was separated into Peak 1 (28 mg, 0.050 mmol) and Example 2 (25 mg, 0.045 mmol) using the following conditions: Chiralcel OD-H (2.0 cm x 25 cm, 5 microns, Chiral Technologies, Inc.), 50% MeOH / EtOH (1:1) / 50% heptane, 20 mL / min flow rate, and UV det...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com