Medicinal composition usable for preventing and/or treating blood coagulation factor ix abnormality, comprising multispecific antigen binding molecule replacing function of blood coagulation factor VIII
A technology of antigen-binding molecules and blood coagulation factors, which can be applied in drug combinations, anticoagulant factor immunoglobulins, peptide/protein components, etc., and can solve the problem of insufficient hemostatic activity to completely stop bleeding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
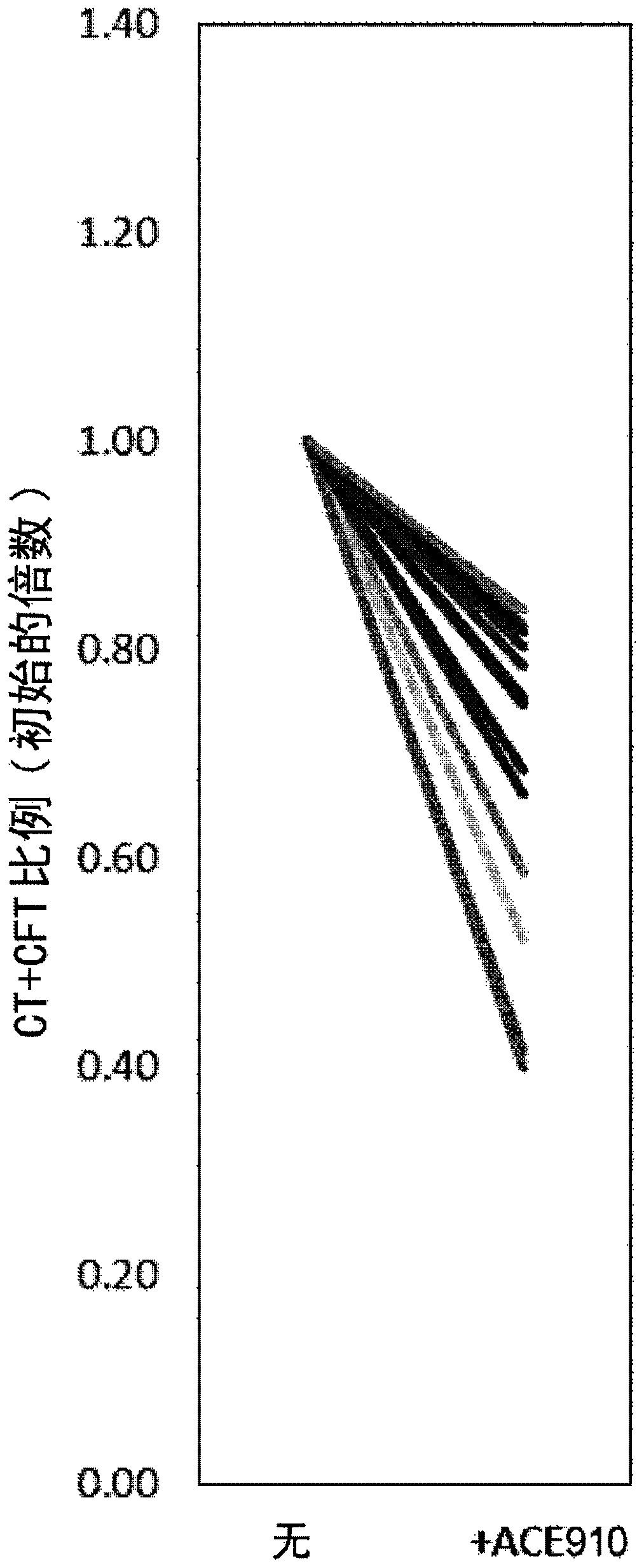
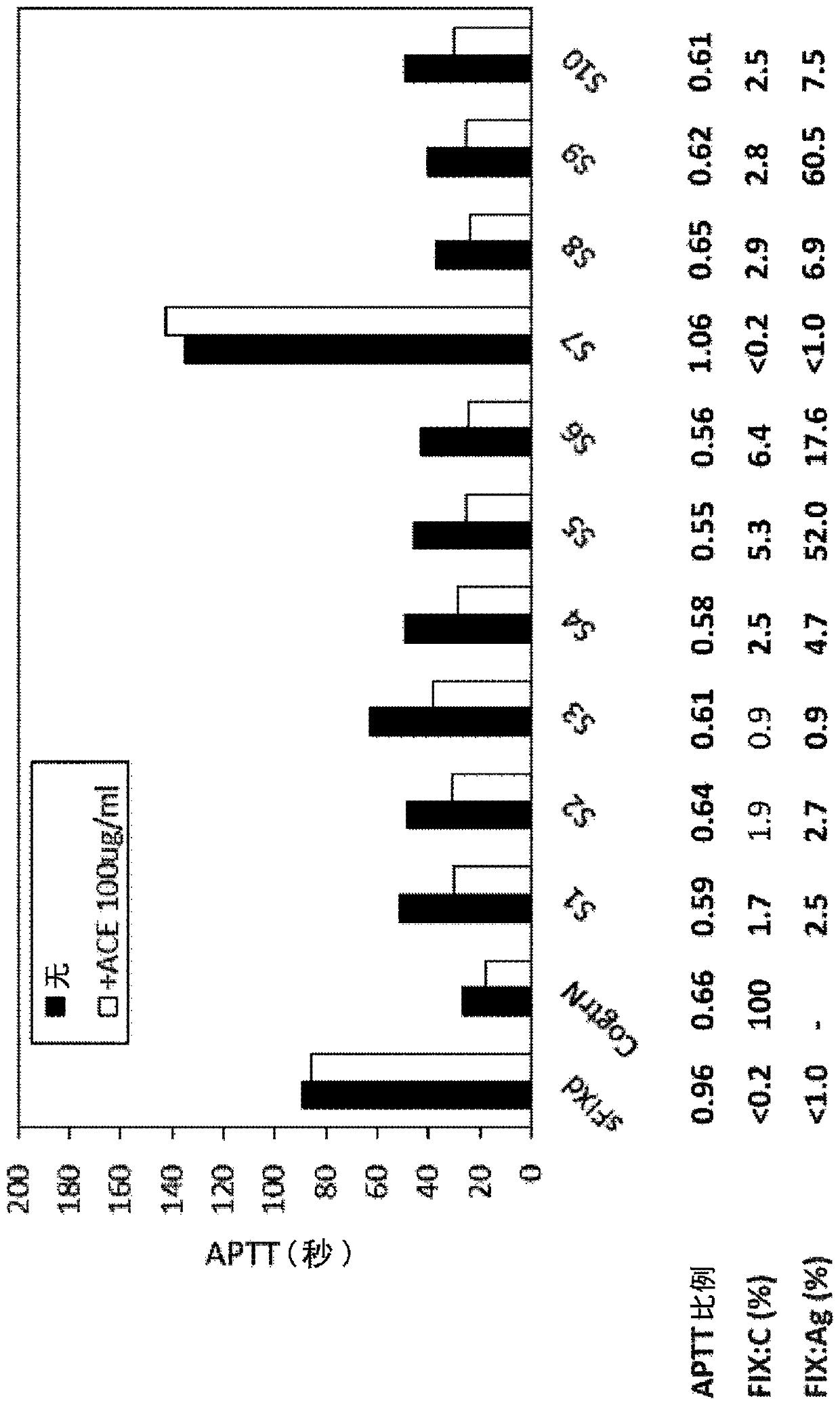
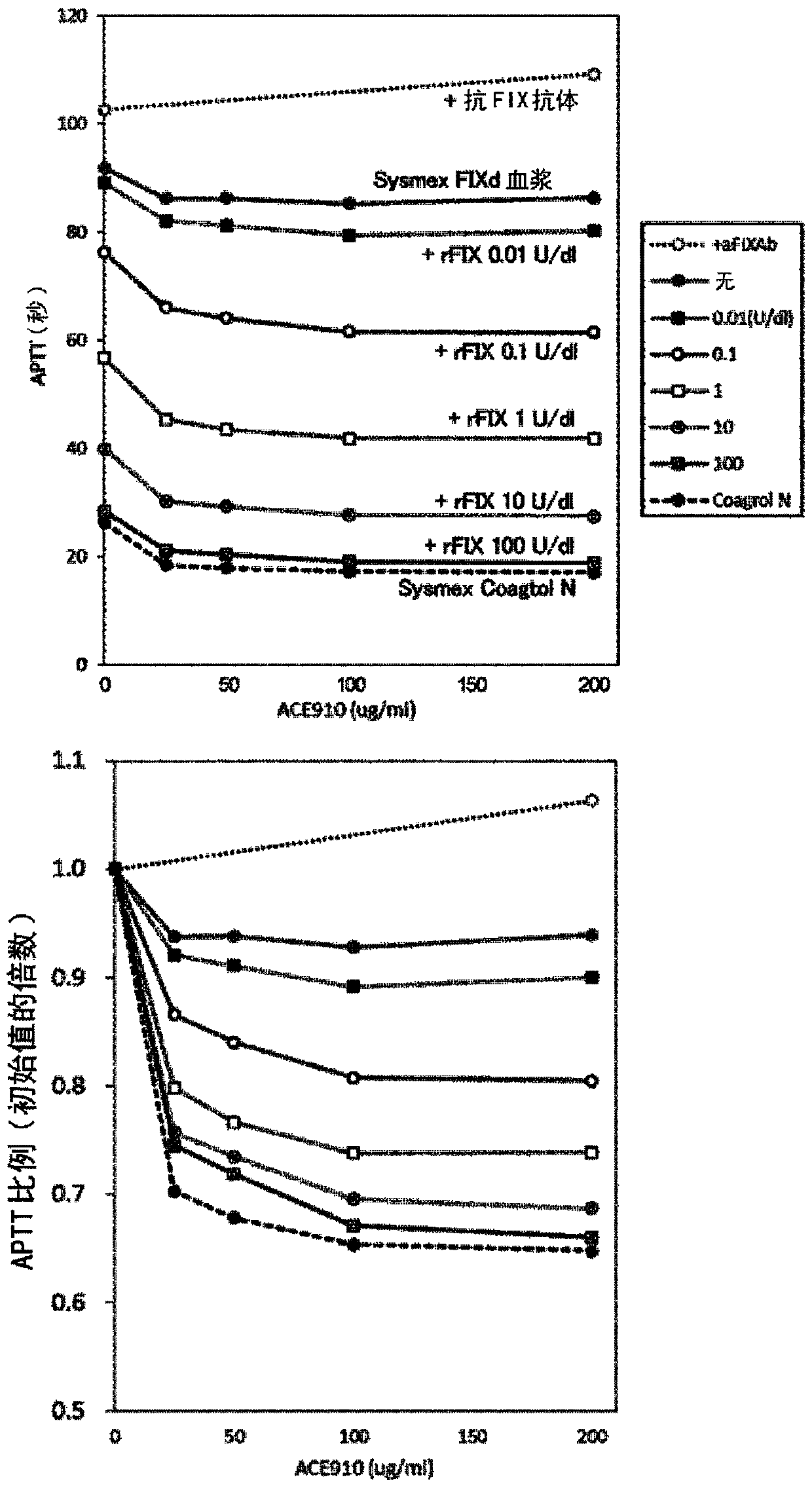
[0198] In the present invention, the use of blood or plasma from FIX disorders other than Hemophilia A, Acquired Hemophilia A, Von Willebrand and Hemophilia C tests functionally replaces multispecific antigen binding of FVIII Blood / plasma procoagulant activity of the molecule. Specifically, using blood / plasma from hemophilia B patients and commercially available FIX-deficient human plasma (GeorgeKing Bio-Medical), ACE910 (Emicerizumab) was examined by each coagulation evaluation method, ROTEM and APTT. Blood / plasma procoagulant activity of anti-(Emicizumab), which is a bispecific antibody described in patent literature (WO2012 / 067176) and is one of the above-mentioned multispecific antigen-binding molecules.
Embodiment 2
[0200] Preparation of ACE910, which is an anti-FIXa / FX bispecific antibody that replaces the function of FⅧ
[0201] ACE910 was obtained by the methods described in WO 2005 / 035756, WO 2006 / 109592 and WO 2012 / 067176. The bispecific antibody was expressed by incorporating the antibody gene into an animal cell expression vector and transfecting it into CHO cells. The bispecific antibody contained in the cell culture supernatant is then purified.
[0202] The FVIII function displacement activity of the bispecific antibodies thus purified was measured by the enzymatic assay shown below. At room temperature, 1 nM human FIXa (Enzyme Research Laboratories), 140 nM human FX (Enzyme Research Laboratories), 20 μM phospholipids (10% phosphatidylserine, 60% phosphatidylcholine, 30% phosphatidylethanolamine) and bispecific antibodies were mixed with Contains 5mM CaCl 2 Mix with 0.1% bovine serum albumin in Tris-buffered saline and incubate for 2 minutes to proceed by FIXa-induced FX ac...
Embodiment 3
[0204] ROTEM measurement
[0205] ROTEM measurements were performed according to conventional methods using a ROTEM delta measuring device (Tem International GmbH). Use calcium solution star-tem reagent (Ref. No. 503-01, Tem International GmbH) as Ca trigger 3002
[0206] APTT measurement
[0207] Thrombocheck APTT-SLA (Sysmex) was used as APTT reagent. 50 μL of APTT reagent was added to 50 μL of FIX disorder patient-derived plasma or FIX-deficient human plasma containing ACE910 and / or anti-FIX-Gla antibodies (Thromb Res 2000; 100:73-79). After incubation at 37°C for 5 minutes, 50 μL of 0.02 mol / L calcium chloride solution was added to initiate the coagulation reaction, and APTT was measured with an automatic blood coagulation measurement device (CS-2000i, Sysmex) according to conventional methods.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com