Improved FIX fusion protein, conjugate and application of fusion protein and conjugate
A technology of fusion protein and conjugate, which is applied in the direction of fusion of polypeptides, peptide/protein components, and prolonging the fusion of plasma life, which can solve the problems of poor physical and chemical stability, limiting patient medication compliance, and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Example 1 Preparation of fusion protein FIX-L1-Fc-L2-P
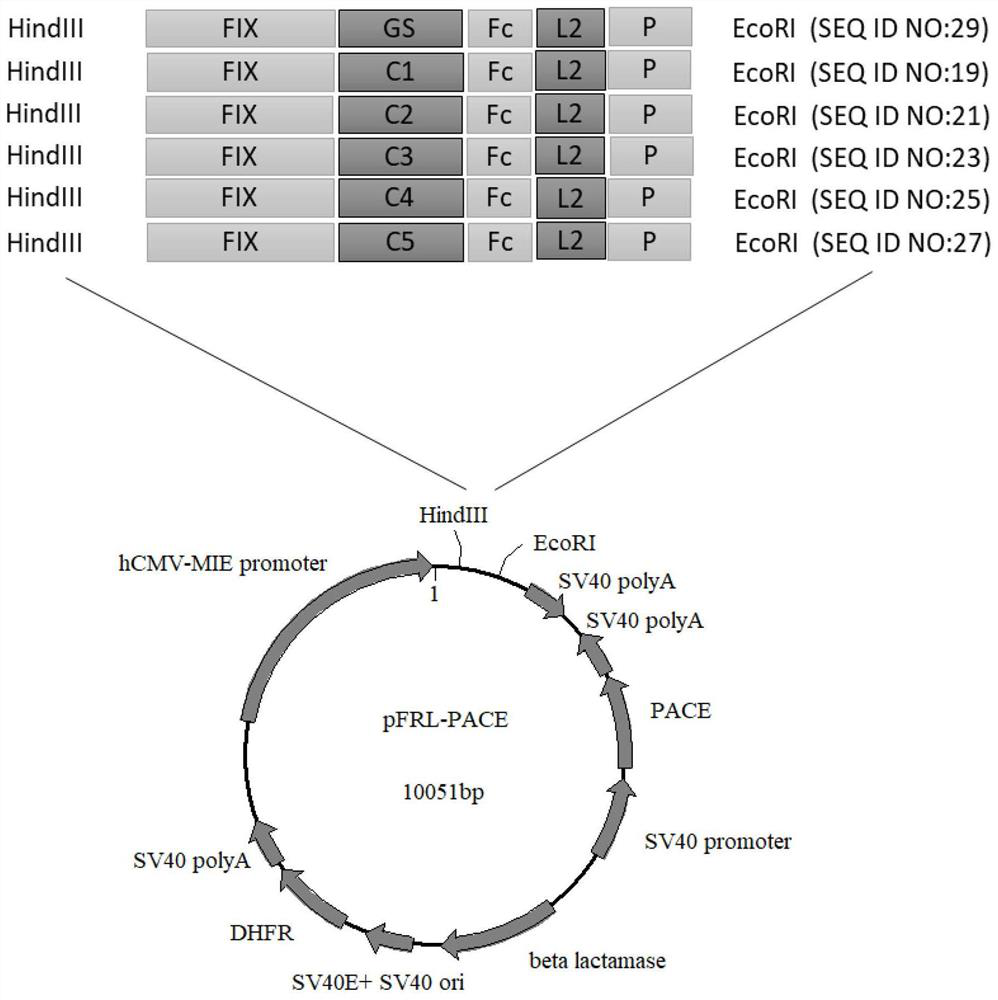
[0127] 1. Construction of expression vector
[0128] The FIX fusion protein constructed in this example has a common molecular structure: FIX-L1-Fc-L2-P. Wherein, FIX represents nine natural blood coagulation factors from human, and its sequence is shown in SEQ ID NO:1.
[0129] L1 represents the first linker between FIX and Fc, and is a connecting peptide composed of a flexible peptide or a flexible peptide and a rigid structure based on multiple prolines (Pro, P). Among them, L1 uses a total of 6 sequences, respectively Represented by C1, C2, C3, C4, C5 and GS, the specific sequence is as follows:
[0130] C1: GGGGSGGGGSGGGGSGGGGSGGGGSVAPPPALPAPVRLPGPA (SEQ ID NO: 8)
[0131] C2: GGGGSGGGGSGGGGSGGGGSGGGGSVAPPPALPAVAPPPALPA (SEQ ID NO: 9)
[0132] C3: GGGGSGGGGSGGGGSGGGGSGGGGSVAPPPALPAVAPPPALPAVAPPPALPAPVRLPGPA (SEQ ID NO: 10)
[0133] C4: GGGGSGGGGSGGGGSGGGGSGGGGSVAPPPALPAPVRLPGPAVAPPPALPAVAPPPALPA (SEQ ID ...
Embodiment 2
[0212] Example 2 In vitro activity detection and comparison of FIX-L1-Fc-L2-P
[0213] This example detects FIX-C1-Fc-L2-P, FIX-C2-Fc-L2-P, FIX-C3-Fc-L2-P, FIX-C4-Fc-L2-P and The in vitro activity of FIX-C5-Fc-L2-P was compared with the in vitro activity of FIX-GS-Fc-L2-P without the rigid unit in the first linker L1.
[0214] The details are as follows: the activity of human coagulation factor IX (hFIX) was determined by a one-phase method based on activated partial thromboplastin time (APTT), with ellagic acid as the activator, and the 8thInternational Standard 2009 FIX Concentrate (WHO FIX Activity standard substance) is carried out APTT determination, takes the logarithm respectively with the activity of known standard substance and measured APTT time, then carries out linear fitting and draws standard curve. Dilute the sample to be tested to the range of the standard curve, mix it with FIX-poor plasma to measure the APPT value, and calculate the biological activity of the ...
Embodiment 3
[0218] Example 3 Preparation of fusion protein FIX-C1-Fc-L2-PEG conjugated with PEG
[0219] The C-terminus of FIX-C1-Fc-L2-P protein contains LPETG, which can be specifically recognized by Sortase A enzyme. Under the action of Sortase A enzyme, the amide bond between T and G is cut off, and T reacts with the sulfhydryl group at position 184 of Sortase enzyme to form a thioester bond intermediate, which is then attacked by GGGAA-PEG with poly-Gly at one end, and finally Able to link PEG with specific molecular weight to the C-terminus of protein.
[0220] PEGlation reaction system: Dissolve 40KD-GGGAA-PEG in buffer and adjust the pH to 7.5, then add protein and 40KD-GGGAA-PEG to the 50mM Tris 150mM NaCl pH 7.5 buffer system at a ratio of 1:20, and then add Sortase A enzyme and 10 mM CaCl 2 After 30 min, EDTA was added to terminate the reaction.
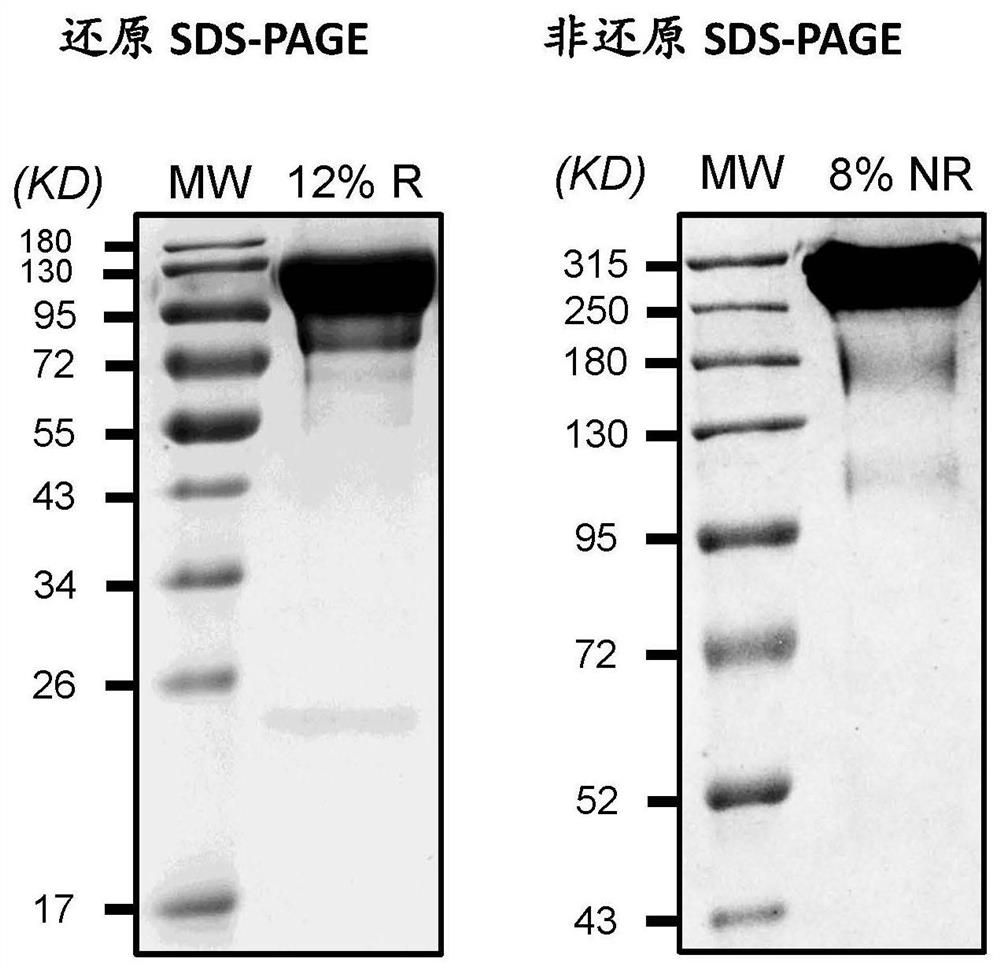
[0221] The conjugation product (FIX-C1-Fc-L2-PEG) was detected by SEC-HPLC for conjugation rate. SEC detection uses TOSOHTSKgel ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Passage density | aaaaa | aaaaa |
| Clearance rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com