Preparation and application of high-activity blood coagulation factor IX mutant, recombinant protein and fusion protein
A blood coagulation factor and mutant protein technology, applied in the field of hemophilia B, can solve the problem of not showing high activity, and achieve the effect of good gene therapy and recombinant protein replacement therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The amino acid sequence of the mutant protein of highly active blood coagulation factor IX is shown in SEQ ID NO:2.
[0034] The preparation method of the mutant protein of highly active blood coagulation factor IX comprises the steps:
[0035] (1) connecting the human coagulation factor IX gene of human wild type or factor IXArg384Gln mutation into the vector to obtain a recombinant vector;
[0036] (2) Transforming the above-mentioned recombinant vector into a host cell to obtain a recombinant strain;
[0037] (3) continuous perfusion culture of the above-mentioned recombinant bacterial strain in serum-free medium to induce the expression of the mutant protein of recombinant highly active blood coagulation factor IX;
[0038] The serum-free medium is "SAFC Biosciences EX-CELL TM 302” (commercialized reagent). In order to ensure product safety and prevent blood-derived preparations from spreading infectious diseases, serum-free medium is used for mammalian cell cult...
Embodiment 2
[0042] The preparation method of the gene therapy AAV vector of highly active blood coagulation factor IX comprises the following steps:
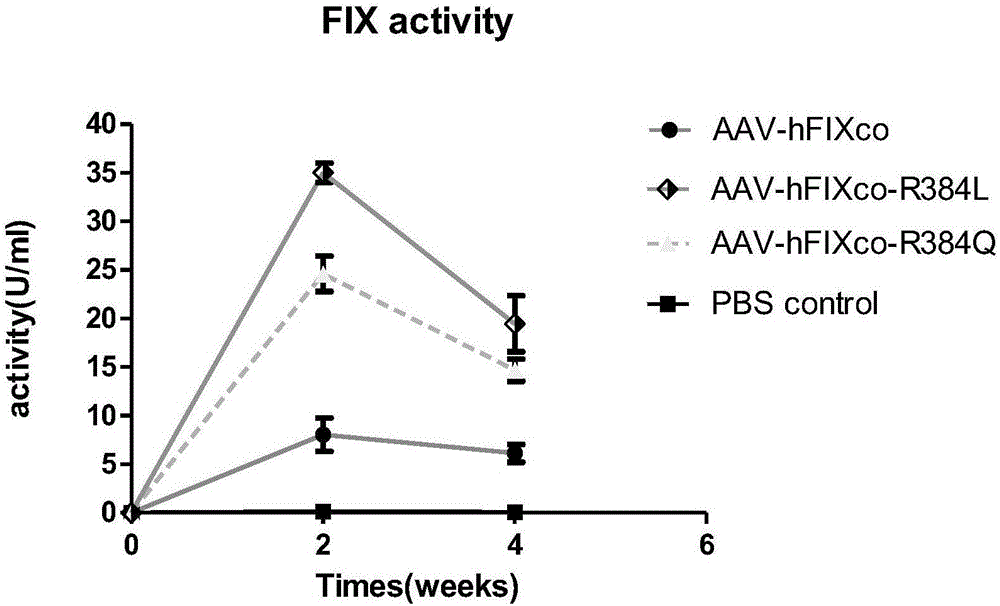
[0043] (1) AAV vectors were prepared using the three-plasmid method. The rAAV-hFIXcoR384Q vector plasmid, the helper plasmid containing AAV Rep / Cap and the helper plasmid containing the adenovirus helper gene were transfected into 293 cells, and then the rAAV-hFIXcoR384Q vector was purified by cesium chloride ultracentrifugation and passed silver staining and quantitative PCR Check the titer. Other vectors rAAV-hFIXcoR384L and rAAV-hFIXco were prepared by the same method.
[0044] (2) The purified rAAV-hFIXR-384Q, rAAV-hFIXco-R384L and rAAV-hFIXco vectors were injected into hemophilia B mice. Select 4-8 week old hemophilic mice, and inject the three vectors into 4x10 via the tail vein 11 Virus particles / ml were injected into 6-7 mice respectively. Three mice were injected with PBS as negative control.
[0045] (3) aPTT detects factor I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com