Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
43 results about "Recombinant factor viii" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Recombinate (recombinant) What is recombinant Recombinate? Recombinate is a naturally occurring protein in the blood that helps blood to clot. A lack of antihemophilic factor VIII is the cause of hemophilia A. Recombinant this medicine works by temporarily raising levels of factor VIII in the blood to aid in clotting.
Preparation of recombinant factor VIII in a protein free medium
InactiveUS6171825B1Eliminate and at least greatly reduce riskImprove productivityFactor VIICulture processFactor iiManganese
Recombinant Factor VIII can be produced in relatively large quantities on a continuous basis from mammalian cells in the absence of any animal-derived proteins such as albumin by culturing the cells in a protein free medium supplemented with polyol copolymers, preferably in the presence of trace metals such as copper. In very preferred embodiments, the medium includes a polyglycol known as Pluronic F-68, copper sulfate, ferrous sulfate / EDTA complex, and salts of trace metals such as manganese, molybdenum, silicon, lithium and chromium. With an alternative medium which included trace copper ions alone (without polyol copolymers) we were also able to enhance the productivity of Factor VIII in recombinant cells such as BHK cells that are genetically engineered to express Factor VIII.
Owner:BAYER HEALTHCARE LLC +1
Recombinant factor VIII having reduced inactivation by activated protein C
ActiveUS8183345B2High catalytic efficiencyPromote localizationFactor VIIBacteriaProtein activationClotting disorders
The present invention relates to a recombinant factor VIII that is characterized by one or more mutations within a region surrounding an activated protein C cleavage site, which one or more mutations result in a reduced rate of inactivation by activated protein C. Isolated nucleic acid molecules, recombinant expression vectors, and host cells suitable for expression of the recombinant factor VIII are also disclosed. The recombinant factor VIII can be used for the treatment of clotting disorders, such as hemophilia A.
Owner:UNIVERSITY OF ROCHESTER
Pharmaceutical preparation of recombinant factor VIII lyophilized without albumin as a stabilizer
ActiveUS6887852B1Same pharmaceutical efficacyAvoid virus infectionFactor VIIPowder deliveryMedicineArginine
Disclosed is a lyophilized preparation of recombinant factor VIII used as a therapeutic preparation of hemophilia A. The lyophilized preparation of recombinant factor VIII is prepared by performing lyophilization using a mixture comprising 6 to 100 mM of L-arginine, 3.5 to 50 mM of L-isoleucine, and 10 to 100 mM of L-glutamic acid as a stabilizer for stabilizing the recombinant factor VIII which exhibits an unstable activity during lyophilization, rather than using human blood derived albumin.
Owner:KOREA GREEN CROSS CORP
Method for purification of factor vii
A method for purifying recombinant Factor VII (rFVII) or recombinant activated Factor VII (rFVIIa), comprising subjecting the rFVII or rFVIIa to liquid chromatography on a hydroxyapatite (HAP) column.
Owner:BAYER HEALTHCARE LLC
Recombinant factor viii having enhanced stability following mutation at the a1-c2 domain interface
InactiveUS20120065136A1Enhance inter-domain binding affinityIncreased stability parameterFactor VIIFungiFactor iiHaemophilia A
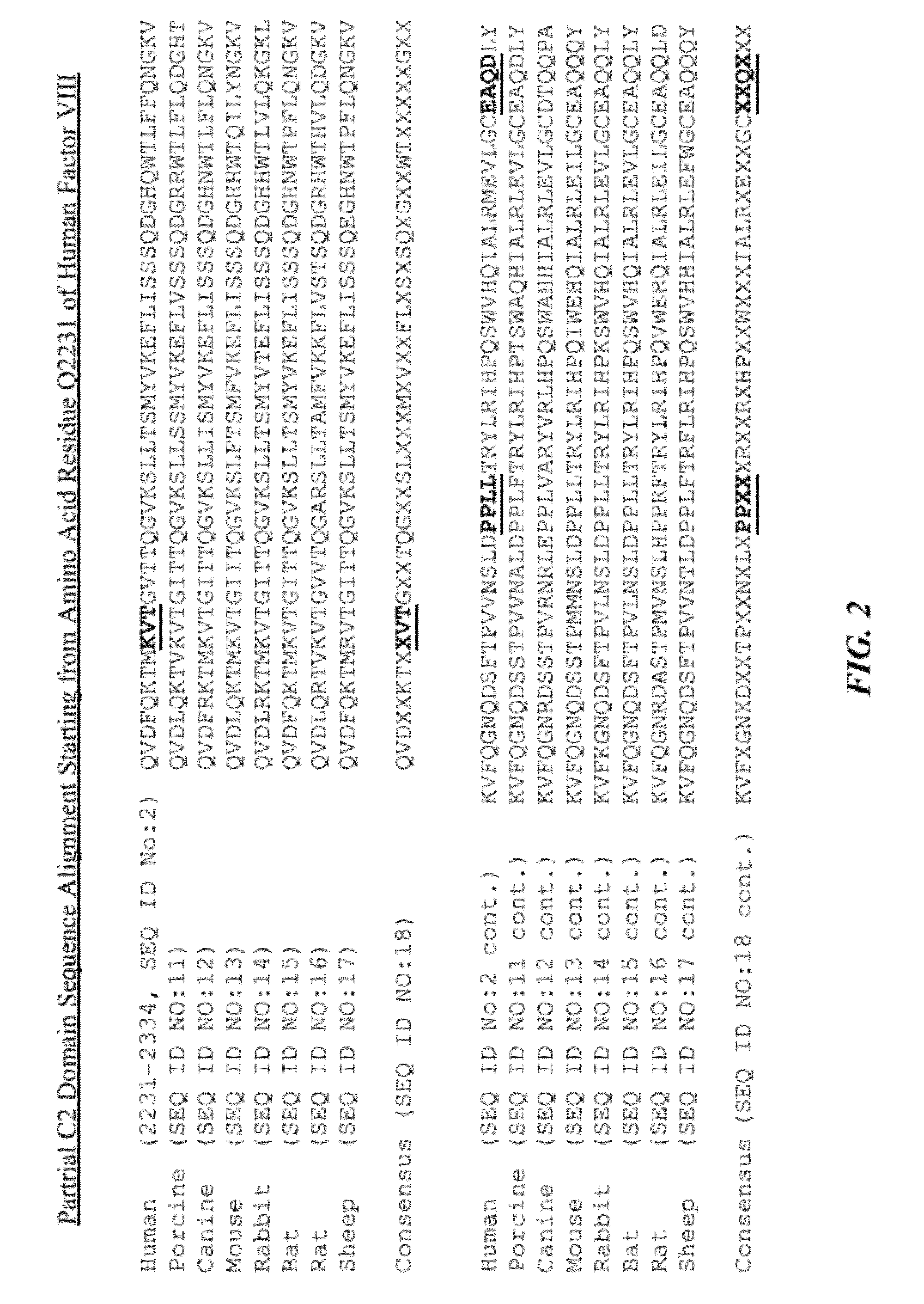
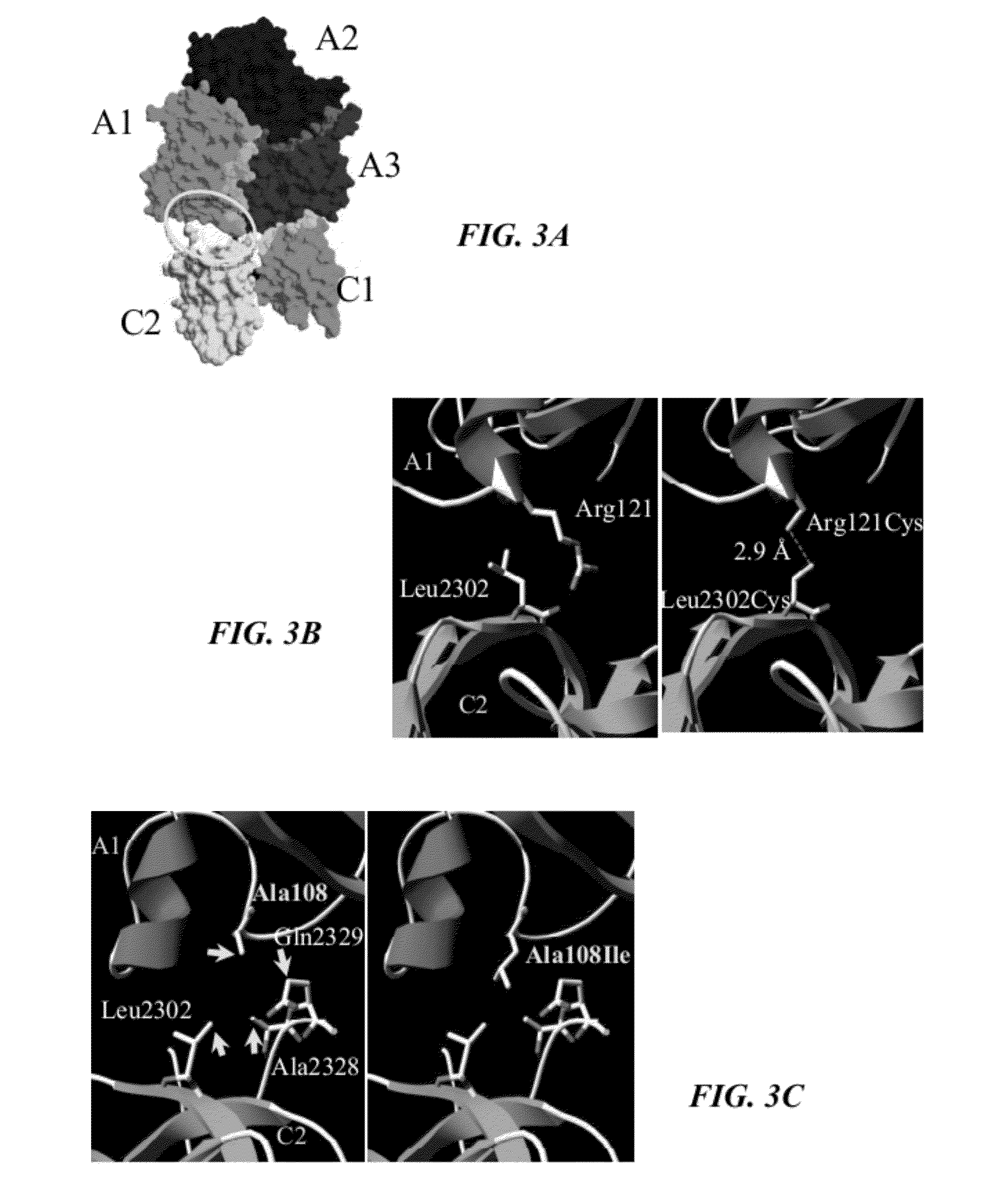
The invention relates to a recombinant factor VIII that includes one or more mutations at an interface of A1 and C2 domains of recombinant factor VIII. The one or more mutations include substitution of one or more amino acid residues with either a cysteine or an amino acid residue having a higher hydrophobicity. This results in enhanced stability of factor VIII. Methods for making the recombinant factor VIII, pharmaceutical compositions containing the recombinant factor VIII, and use of the recombinant factor VIII for treating hemophilia A are also disclosed.
Owner:UNIVERSITY OF ROCHESTER
Recombinant factor viii having reduced inactivation by activated protein c
ActiveUS20090118185A1High catalytic efficiencyPromote localizationFactor VIIFungiProtein activationFactor ii
The present invention relates to a recombinant factor VIII that is characterized by one or more mutations within a region surrounding an activated protein C cleavage site, which one or more mutations result in a reduced rate of inactivation by activated protein C. Isolated nucleic acid molecules, recombinant expression vectors, and host cells suitable for expression of the recombinant factor VIII are also disclosed. The recombinant factor VIII can be used for the treatment of clotting disorders, such as hemophilia A.
Owner:UNIVERSITY OF ROCHESTER
Recombinant Factor VIII Having Increased Specific Activity
InactiveUS20070265199A1Great procoagulant activityReduce dosageFactor VIIPeptide/protein ingredientsFactor iiWild type
The present invention relates to recombinant factor VIII having a specific activity that is higher than that of the corresponding wild-type factor VIII. The present invention also relates to methods of making and using the recombinant factor VIII. The present invention also relates to an isolated nucleic acid molecule that encodes the recombinant factor VIII, as well as DNA expression systems and host cells containing the isolated nucleic acid molecule.
Owner:UNIVERSITY OF ROCHESTER
Recombinant factor viii having increased stability
The present invention relates to a recombinant factor VIII that includes one or more mutations that result in enhanced stability of both factor VIII and factor VIIIa. Methods of making and using the recombinant factor VIII, and pharmaceutical compositions containing the same are also disclosed. The present invention further relates to an isolated nucleic acid molecule that encodes the recombinant factor VIII, as well as DNA expression systems and host cells containing the isolated nucleic acid molecule.
Owner:UNIVERSITY OF ROCHESTER
Modified recombinant factor VIII and von Willebrand factor and methods of use
Owner:TAKEDA PHARMA CO LTD
Industrial-scale Serum-free Production of Recombinant Factor VII in Mammalian Cells
The invention provides a method for industrial-scale production of FVII polypeptides in mammalian cell culture free of animal-derived components.
Owner:NOVO NORDISK AS
Recombinant factor VIII binding peptides
Peptides that have domains that bind to recombinant factor VIII (rFVIII) are disclosed. A method of rFVIII binding assay using the peptides deduced from a combinatorial library in a filtration plate process is described. A method of using peptides having these available binding domains in an affinity chromatography process to purify factor VIII is also taught.
Owner:BAYER CORPORATION +1
Recombinant factor VIII having increased specific activity
InactiveUS7855274B2High activityLower medical costsFactor VIIPeptide/protein ingredientsWild typeFactor ii
The present invention relates to recombinant factor VIII having a specific activity that is higher than that of the corresponding wild-type factor VIII. The present invention also relates to methods of making and using the recombinant factor VIII. The present invention also relates to an isolated nucleic acid molecule that encodes the recombinant factor VIII, as well as DNA expression systems and host cells containing the isolated nucleic acid molecule.
Owner:UNIVERSITY OF ROCHESTER
Recombinant human blood coagulation factor VIII protein, composition, use of a recombinant factor VIII protein, use of a composition, method of obtaining a recombinant human blood coagulation factor VIII protein and use thereof
InactiveUS20100172891A1Improve biological activityImprove the level ofFactor VIIPeptide/protein ingredientsHemophiliasHuman Blood Coagulation Factor
The present invention refers to a recombinant human blood coagulation factor VIII protein and a composition containing it. The present invention also refers to the use of the protein or composition of the invention for manufacturing a medicine for treating hemophilia A. Additionally, the present invention refers to the method of obtaining a recombinant human blood coagulation factor VIII protein. A further object of the present invention is a recombinant protein obtained by the method described herein, and its use in the preparation of a medicine for the treatment of hemophilia A.
Owner:FUNDACAO HEMOCENT DE RIBEIRAO PRETO +1
Pharmaceutical preparation of recombinant factor VIII lyophilized without albumin as a stabilizer
Disclosed is a lyophilized preparation of recombinant factor VIII used as a therapeutic preparation of hemophilia A. The lyophilized preparation of recombinant factor VIII is prepared by performing lyophilization using a mixture comprising 6 to 100 mM of L-arginine, 3.5 to 50 mM of L-isoleucine, and 10 to 100 mM of L-glutamic acid as a stabilizer for stabilizing the recombinant factor VIII which exhibits an unstable activity during lyophilization, rather than using human blood derived albumin.
Owner:GREEN CROSS CORP THE
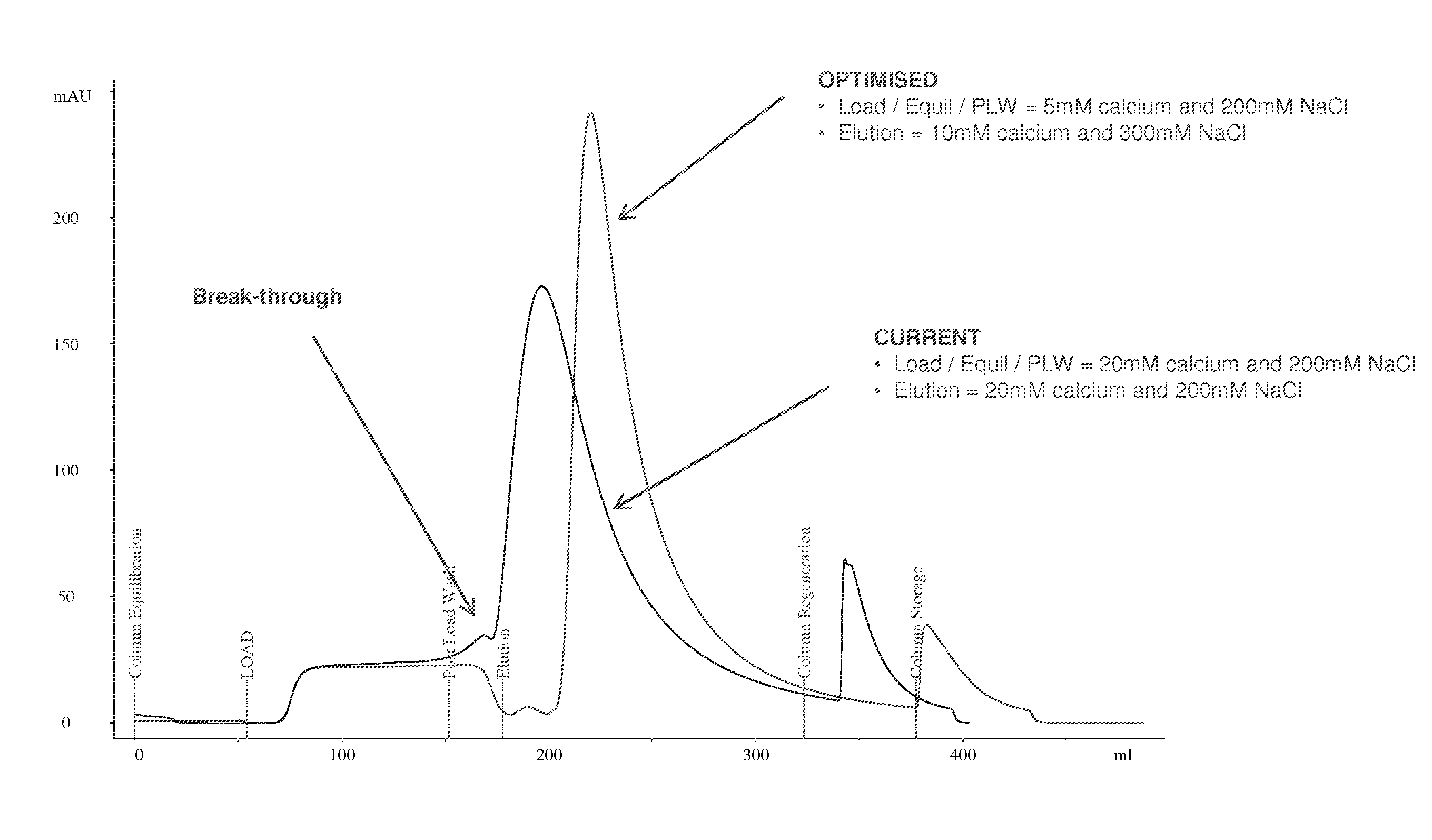
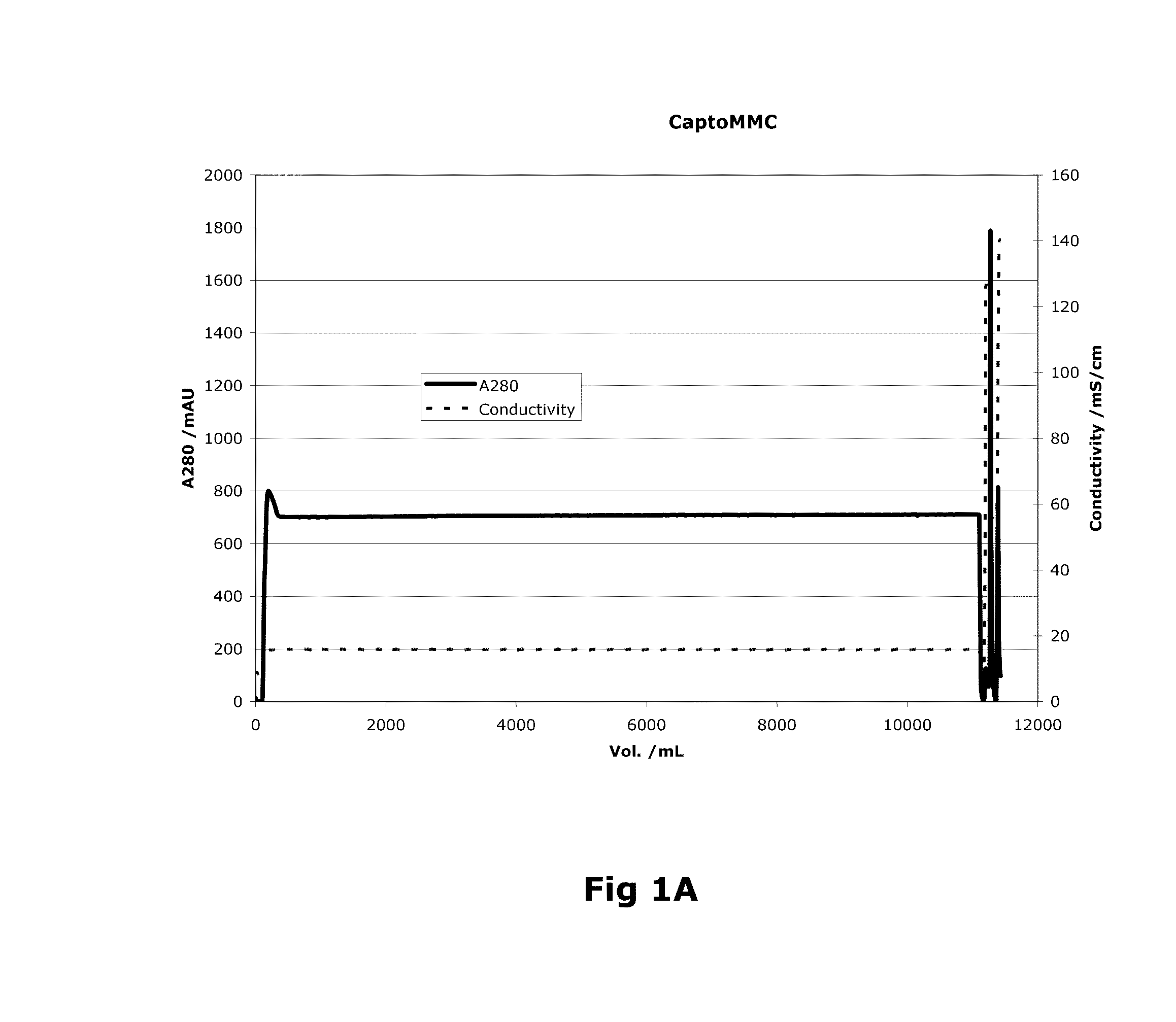
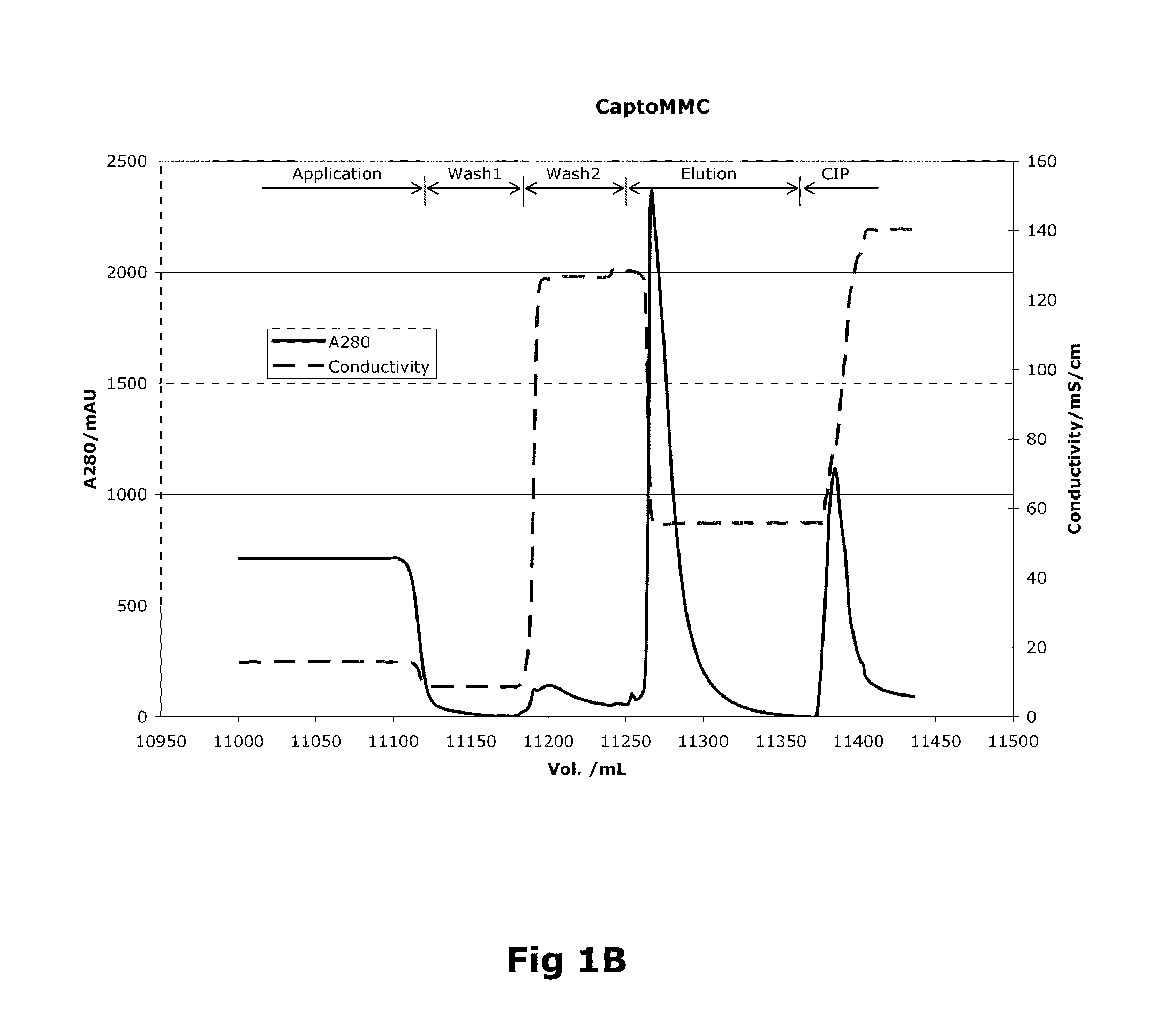
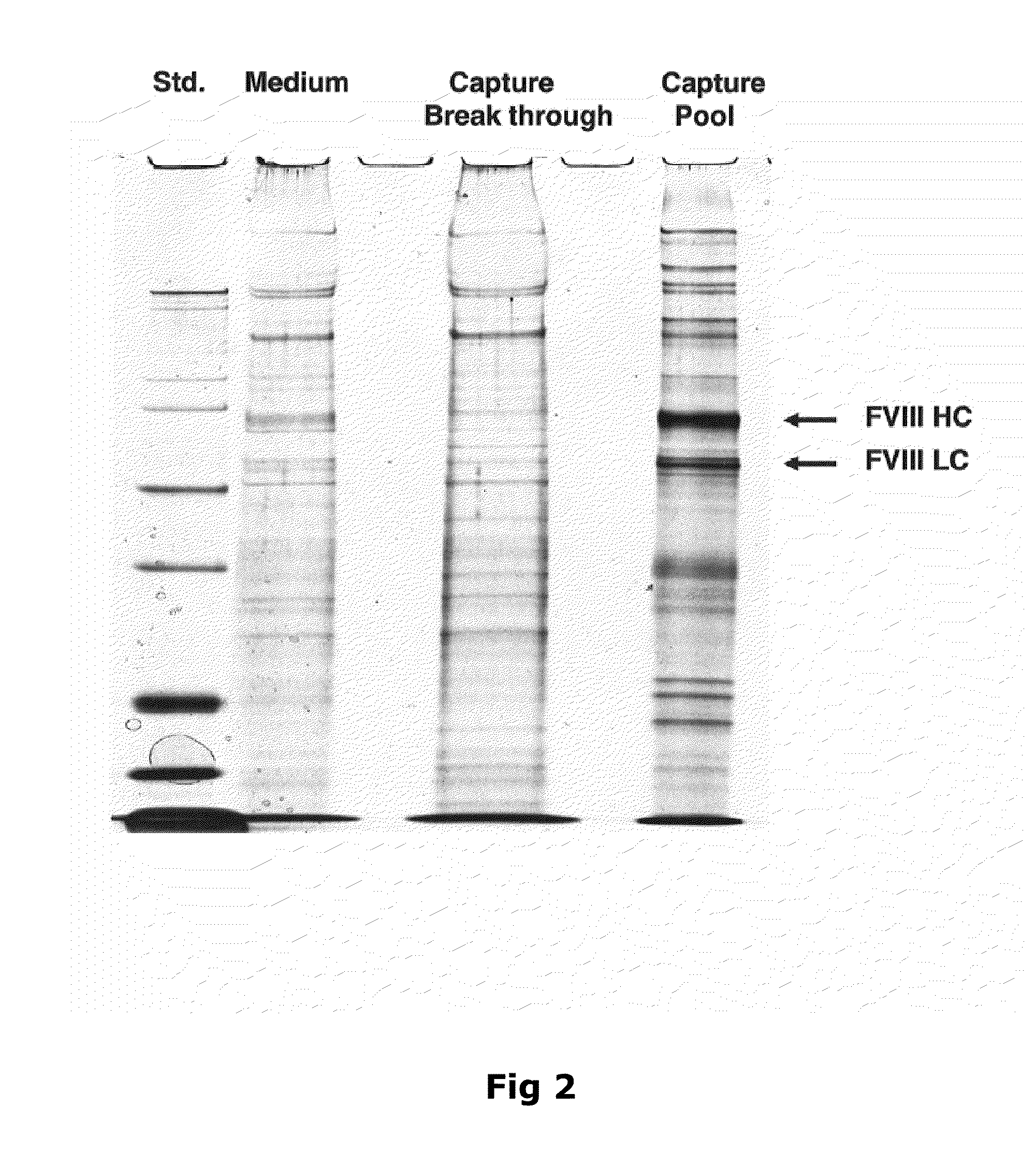
Purification of Factor VIII Using a Mixed-Mode or Multimodal Resin
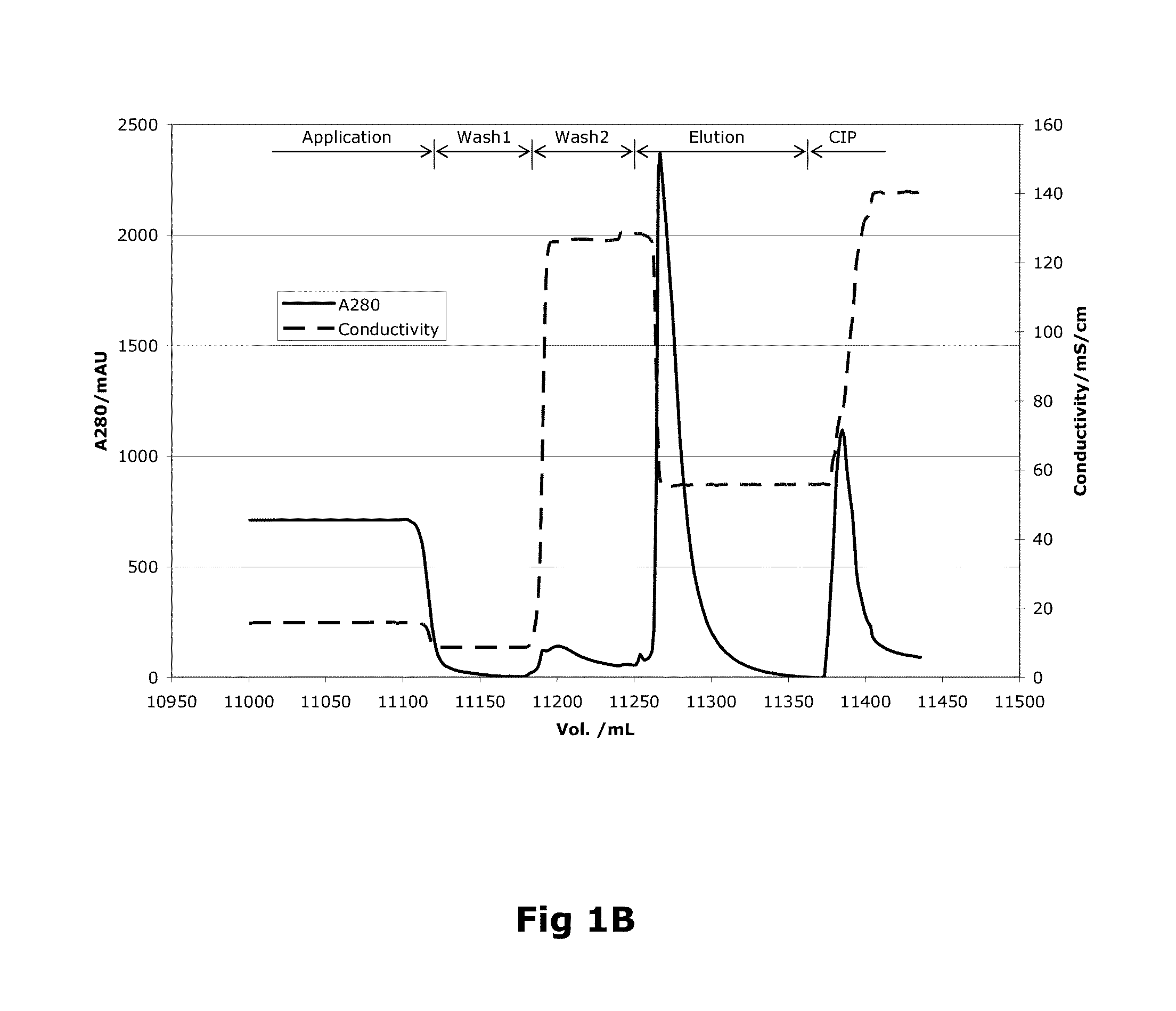
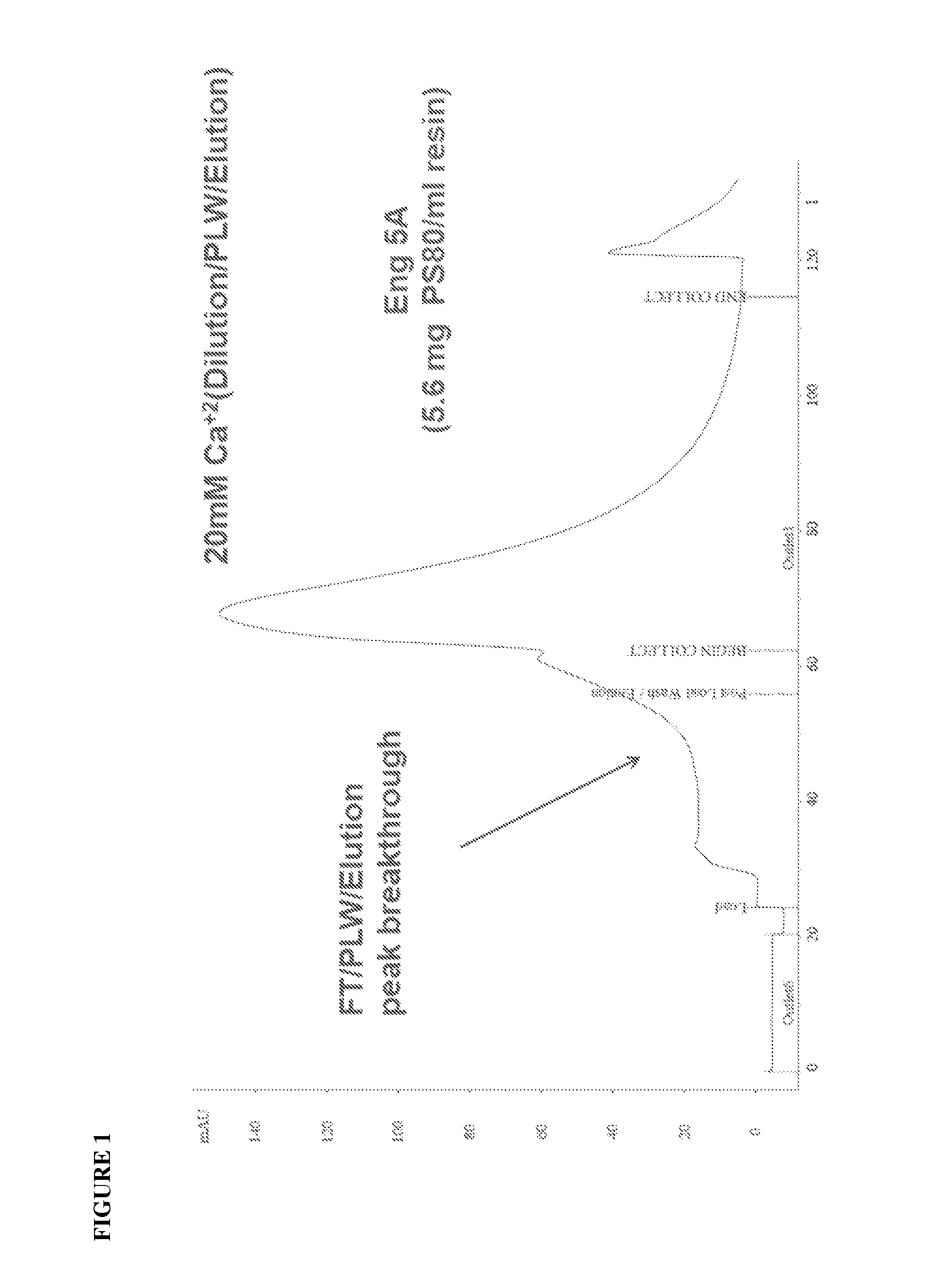
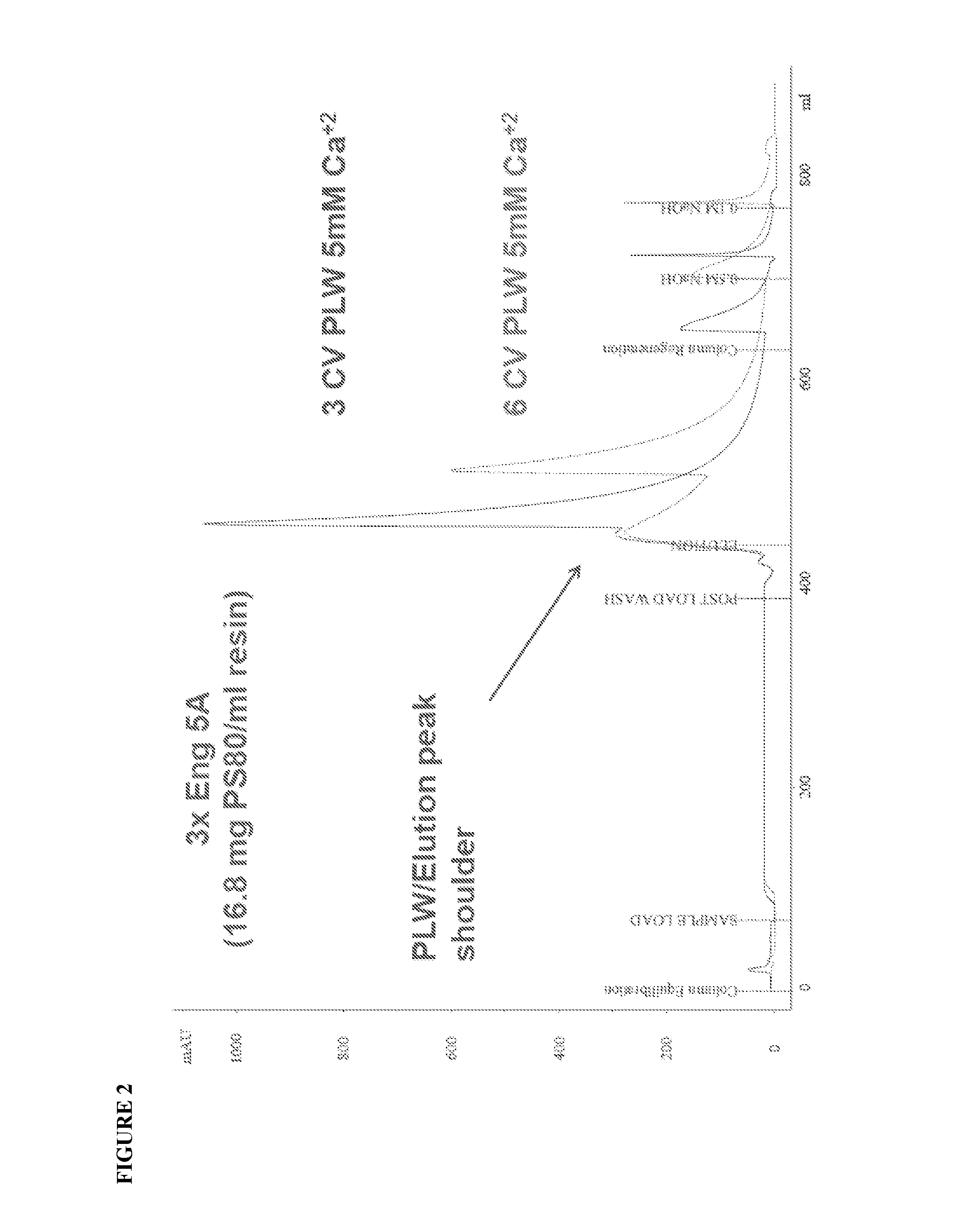
ActiveUS20100204452A1Fast and efficient purificationWithout loss activityFactor VIIMammal material medical ingredientsLoad stepClotting factor
A method for purifying a recombinant protein using a multimodal or mixed mode resin containing ligands which comprise a hydrophobic part and a negatively charged part is described. The invention is advantageous in that it is a single step chromatographic process which does not require adjustment of pH or conductivity during loading step and results in high yield and potency. The process is used for the purification of recombinant compositions of coagulation factor, particularly recombinant Factor VIII.
Owner:NOVO NORDISK AS
Factor VIII Molecules With Reduced VWF Binding
InactiveUS20130040888A1Reduced vWF binding capacityFactor VIIPeptide/protein ingredientsOrganic chemistryRecombinant factor viii
The present invention relates to a recombinant Factor VIII molecule, wherein said molecule has reduced vWF binding capacity, and wherein said molecule is covalently conjugated with at least one side group.
Owner:NOVO NORDISK AS
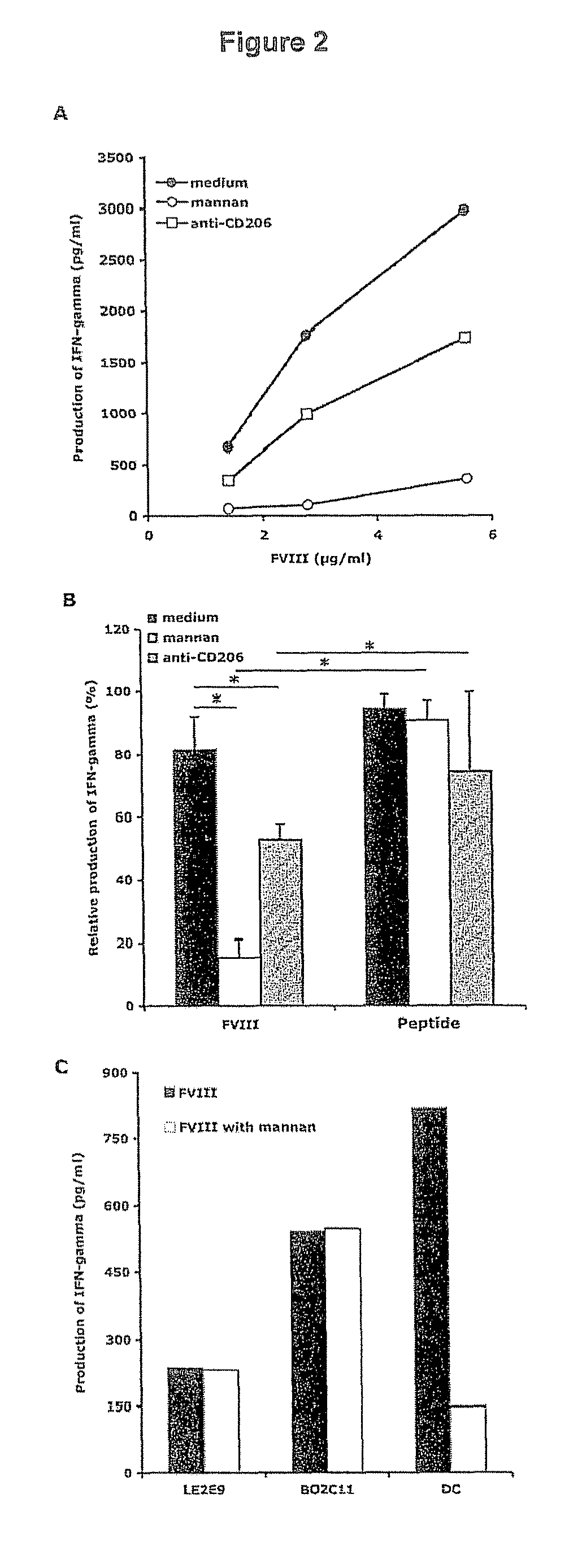
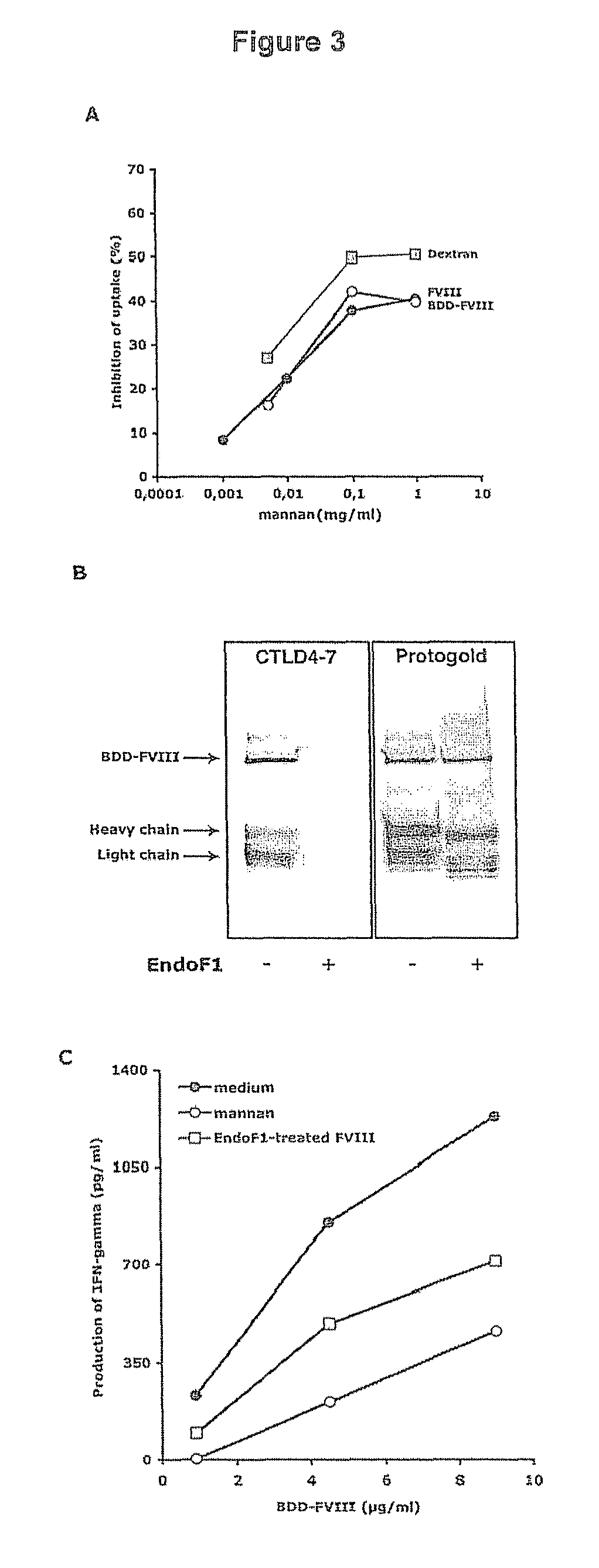
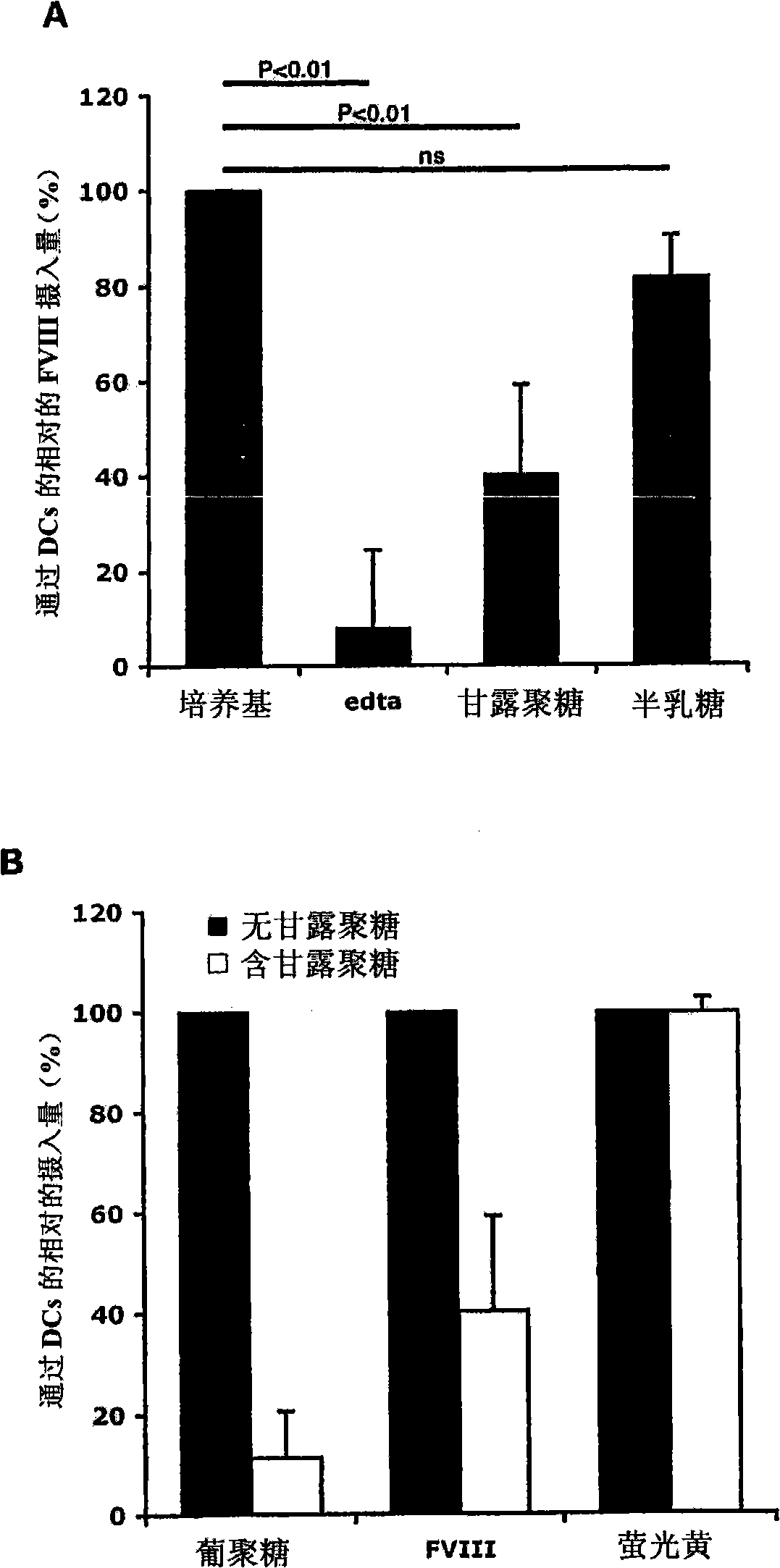
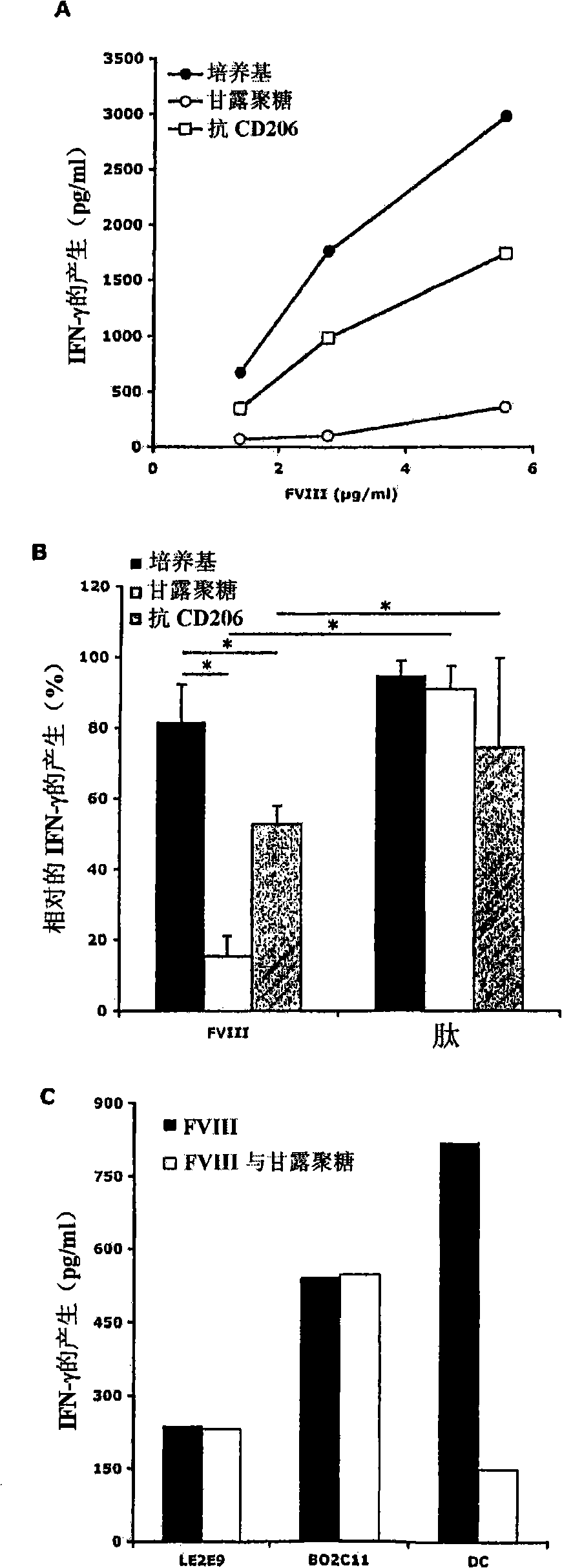
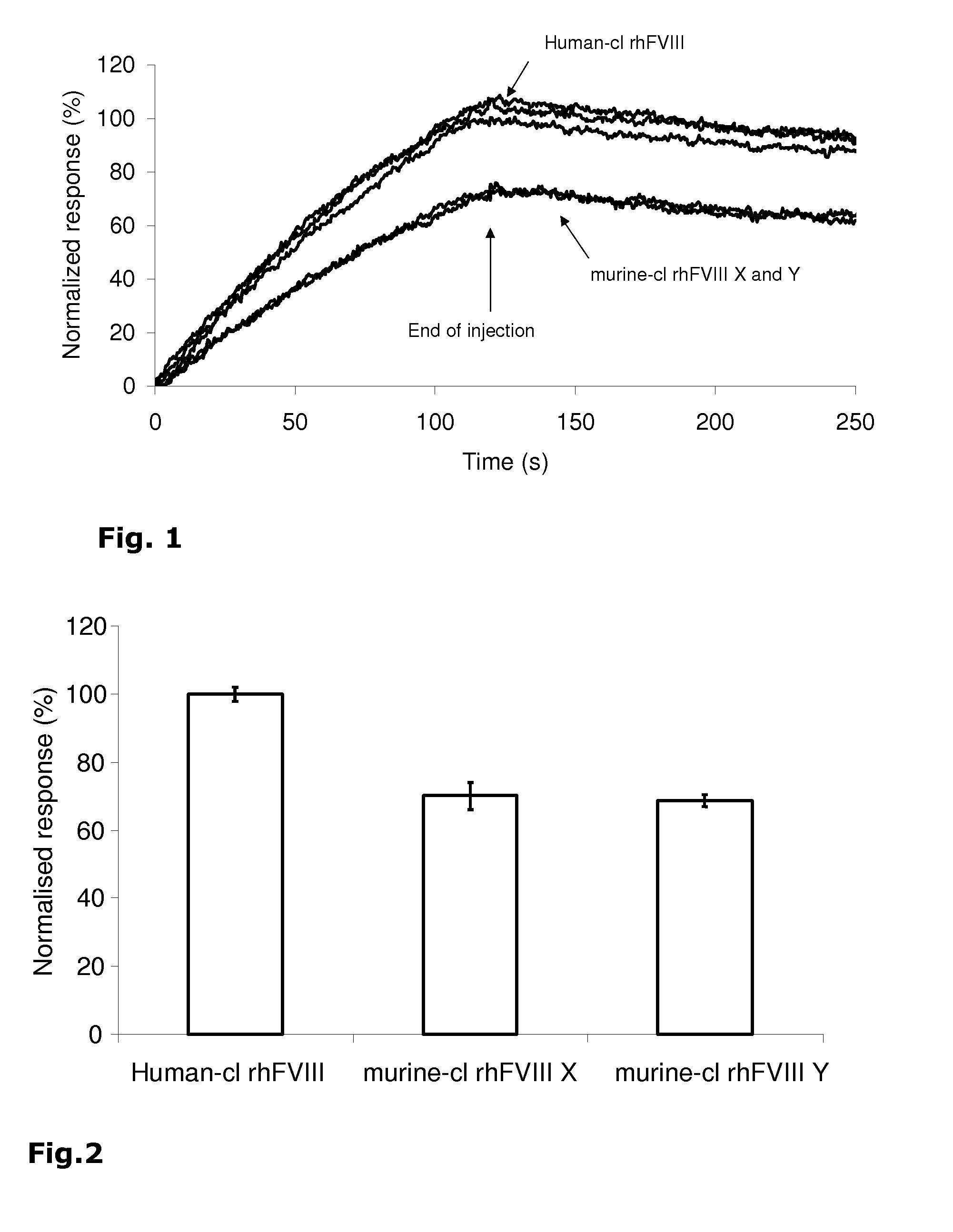
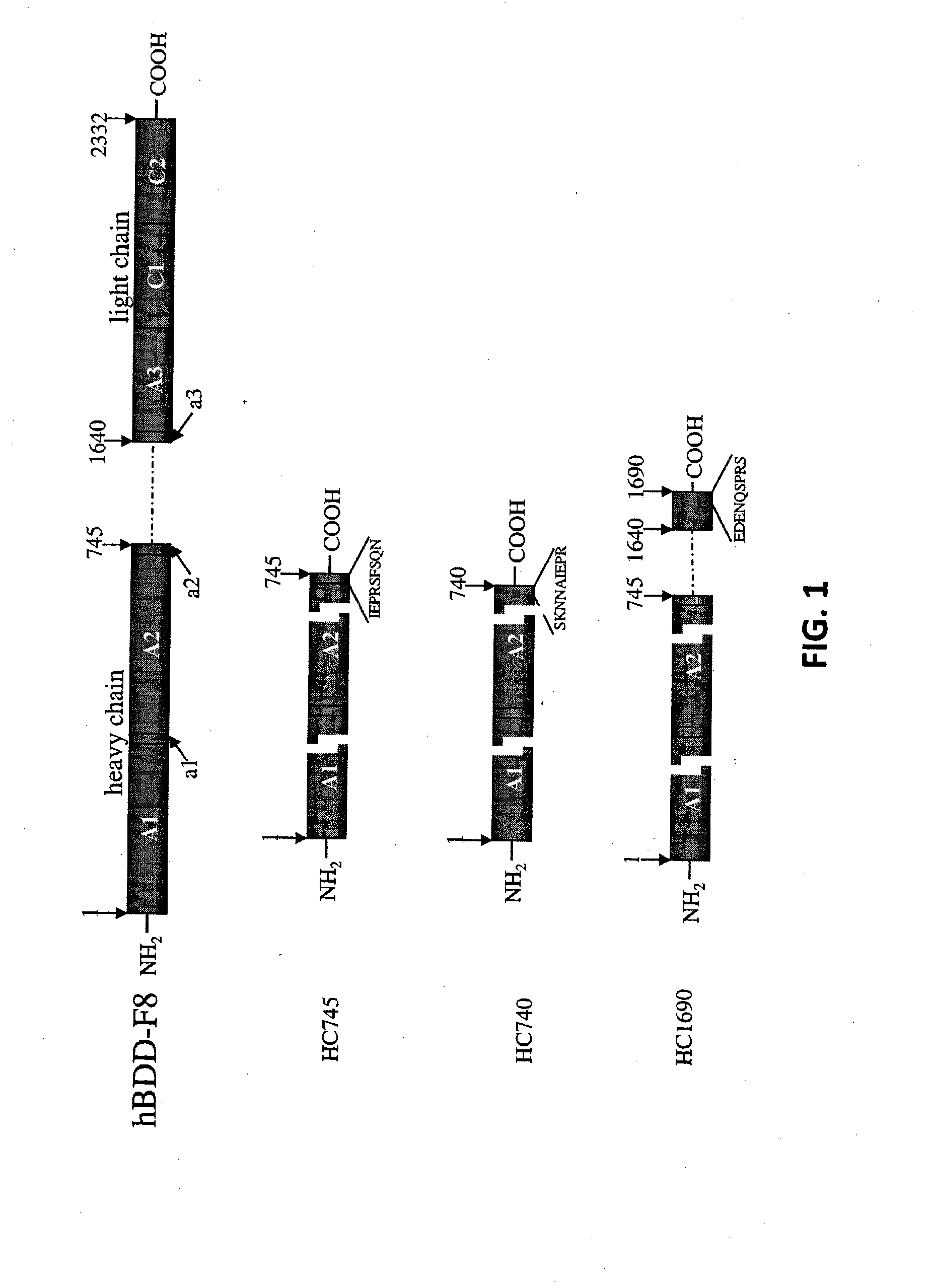
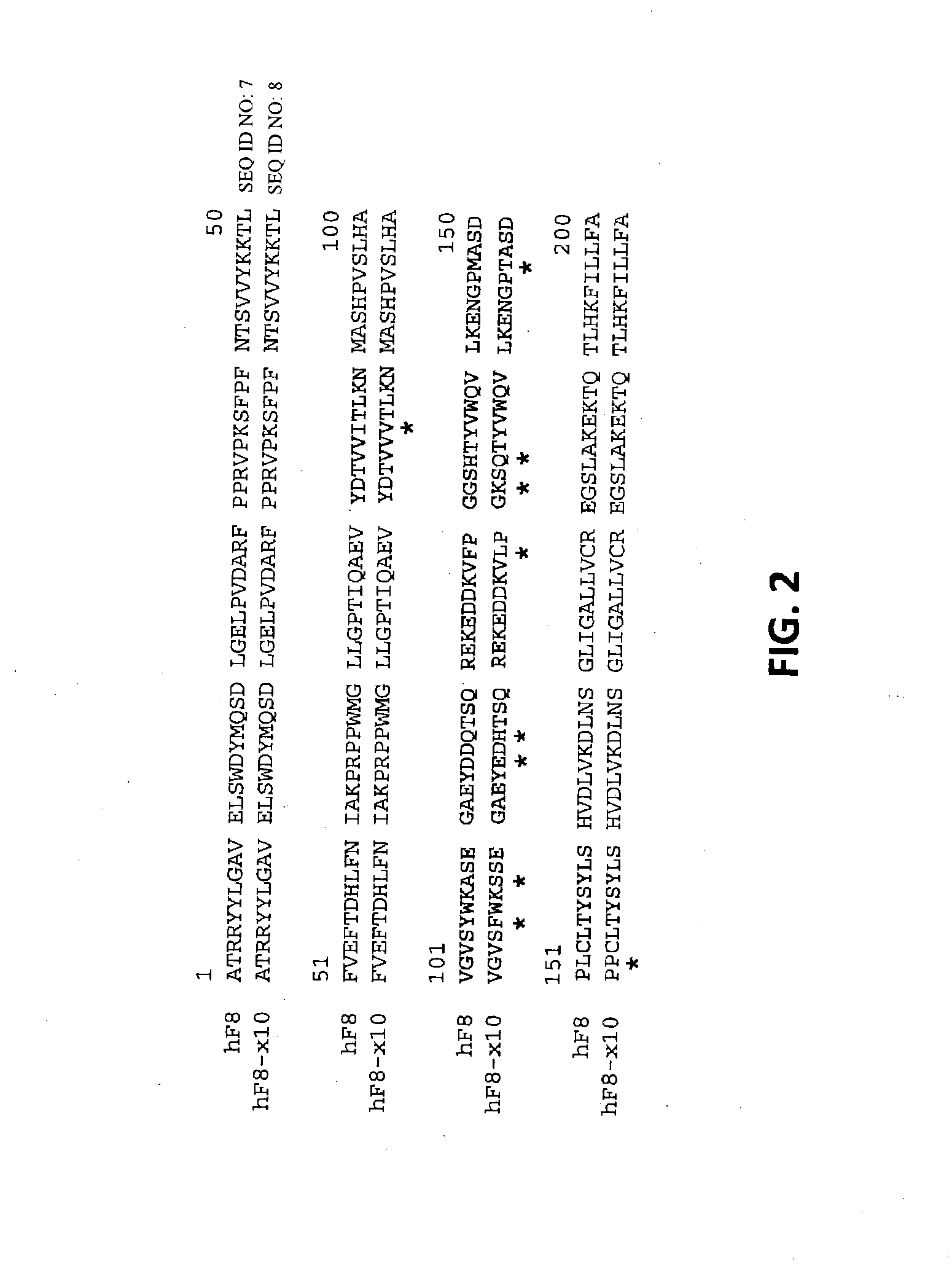
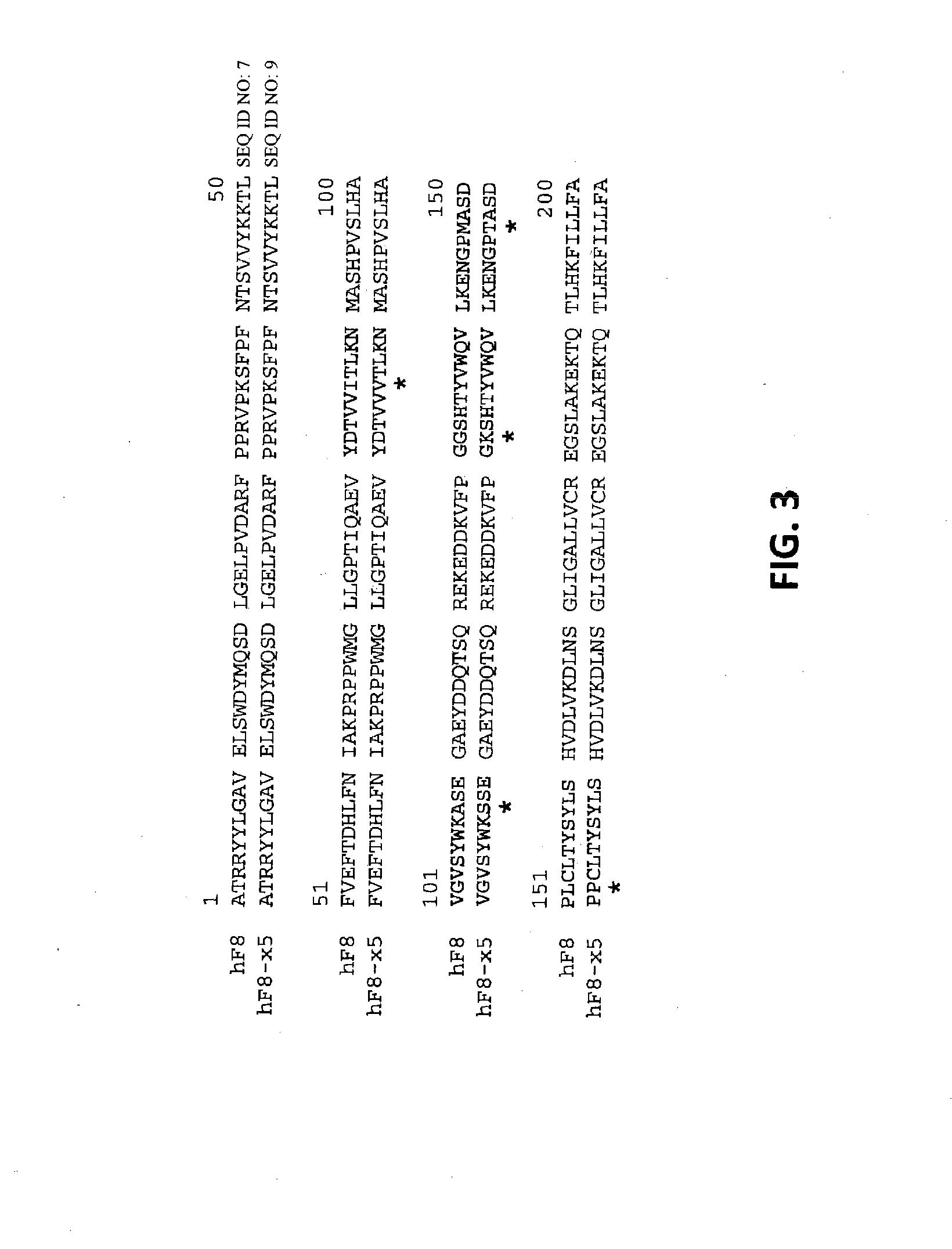
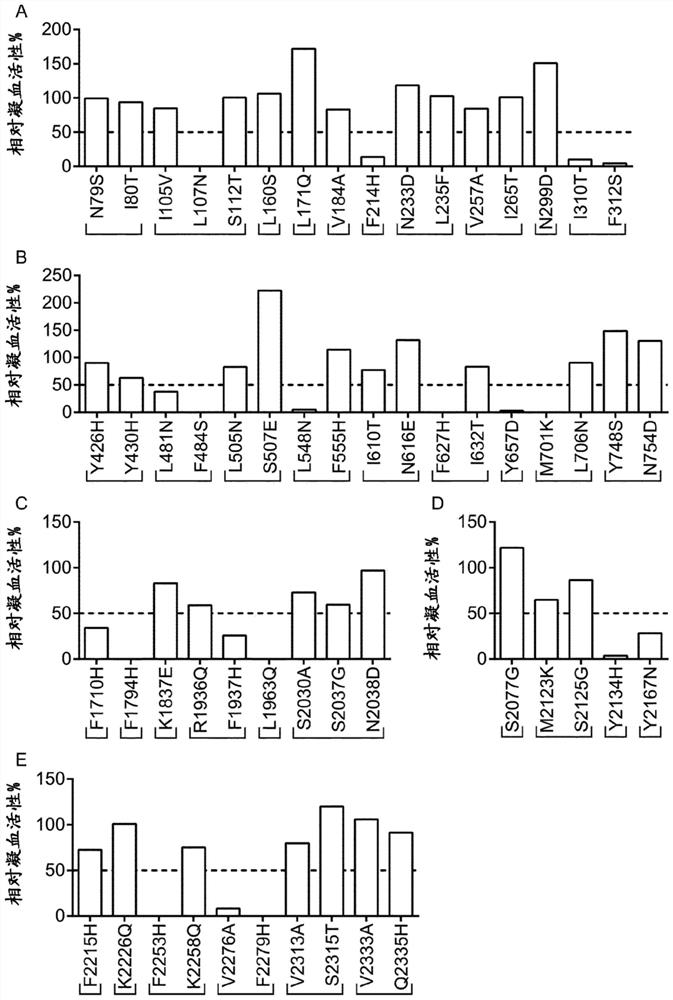
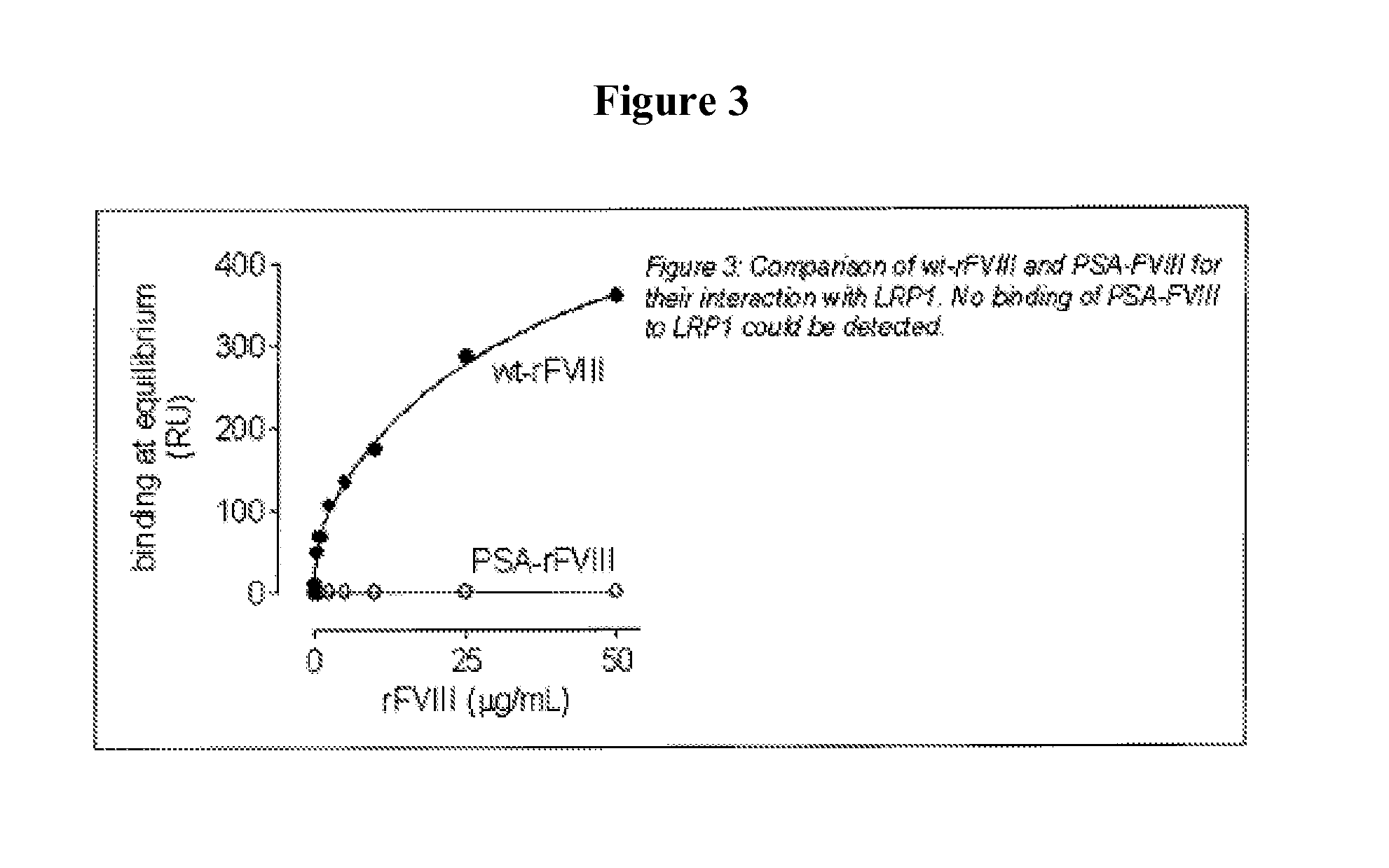
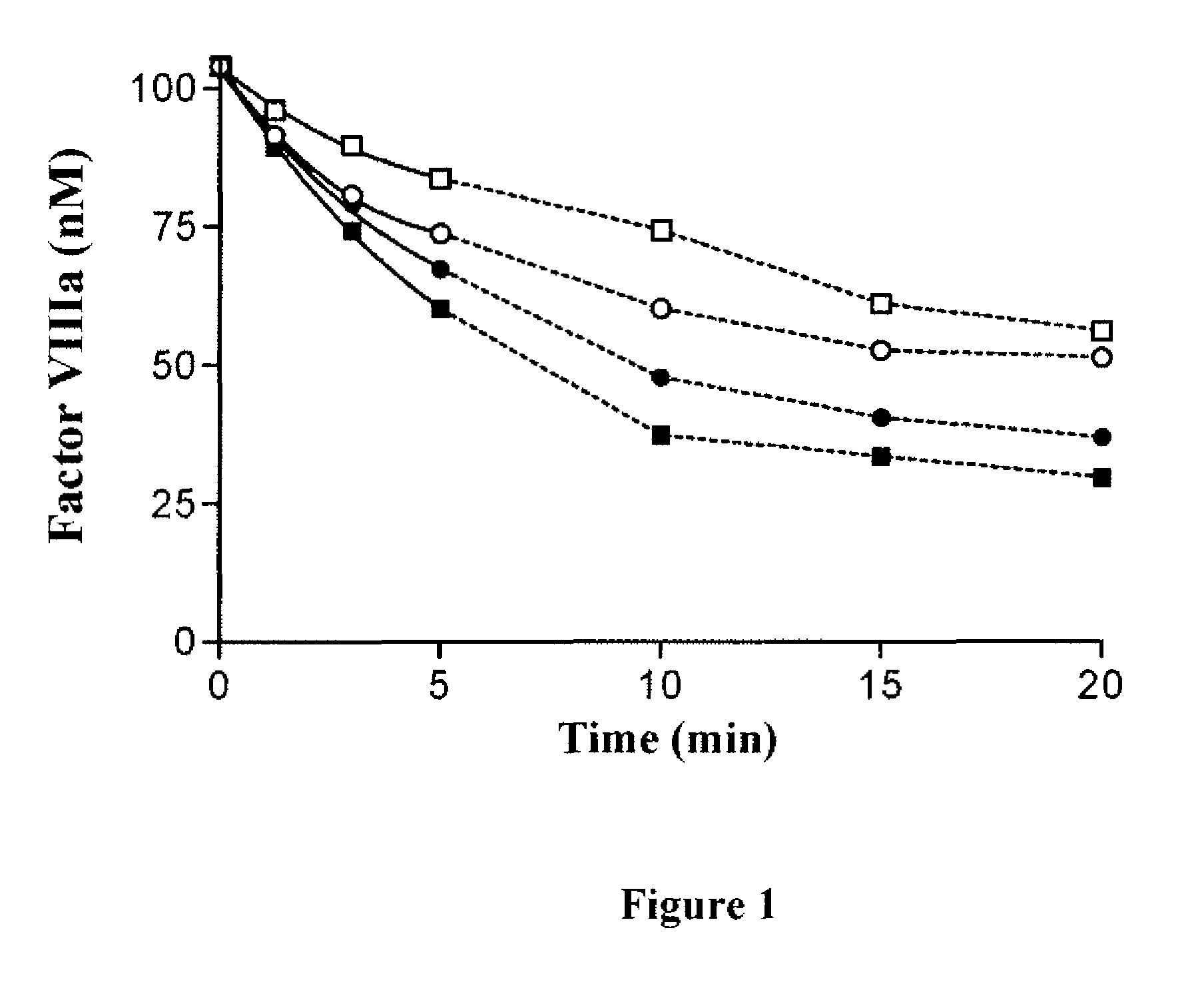
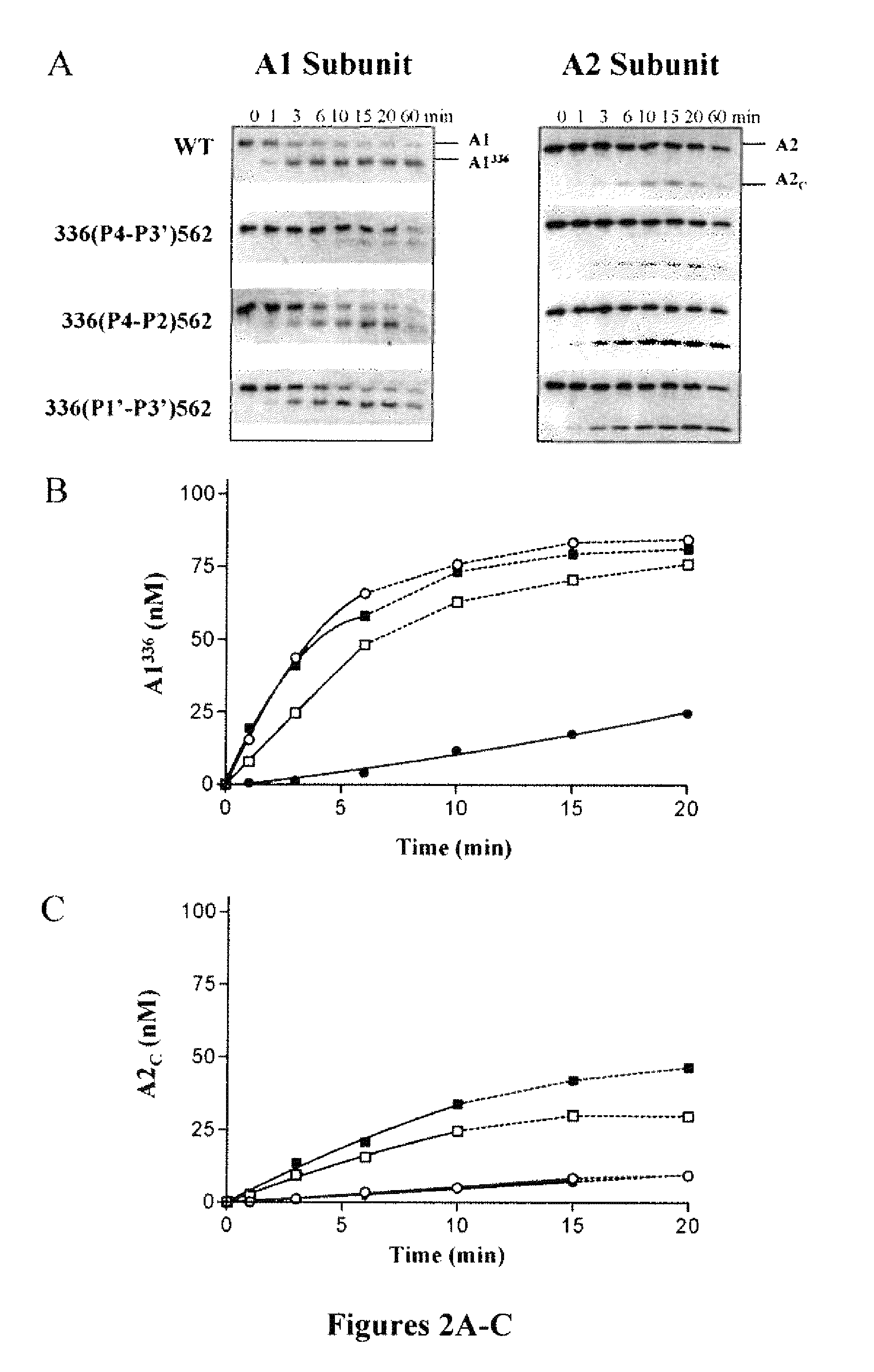
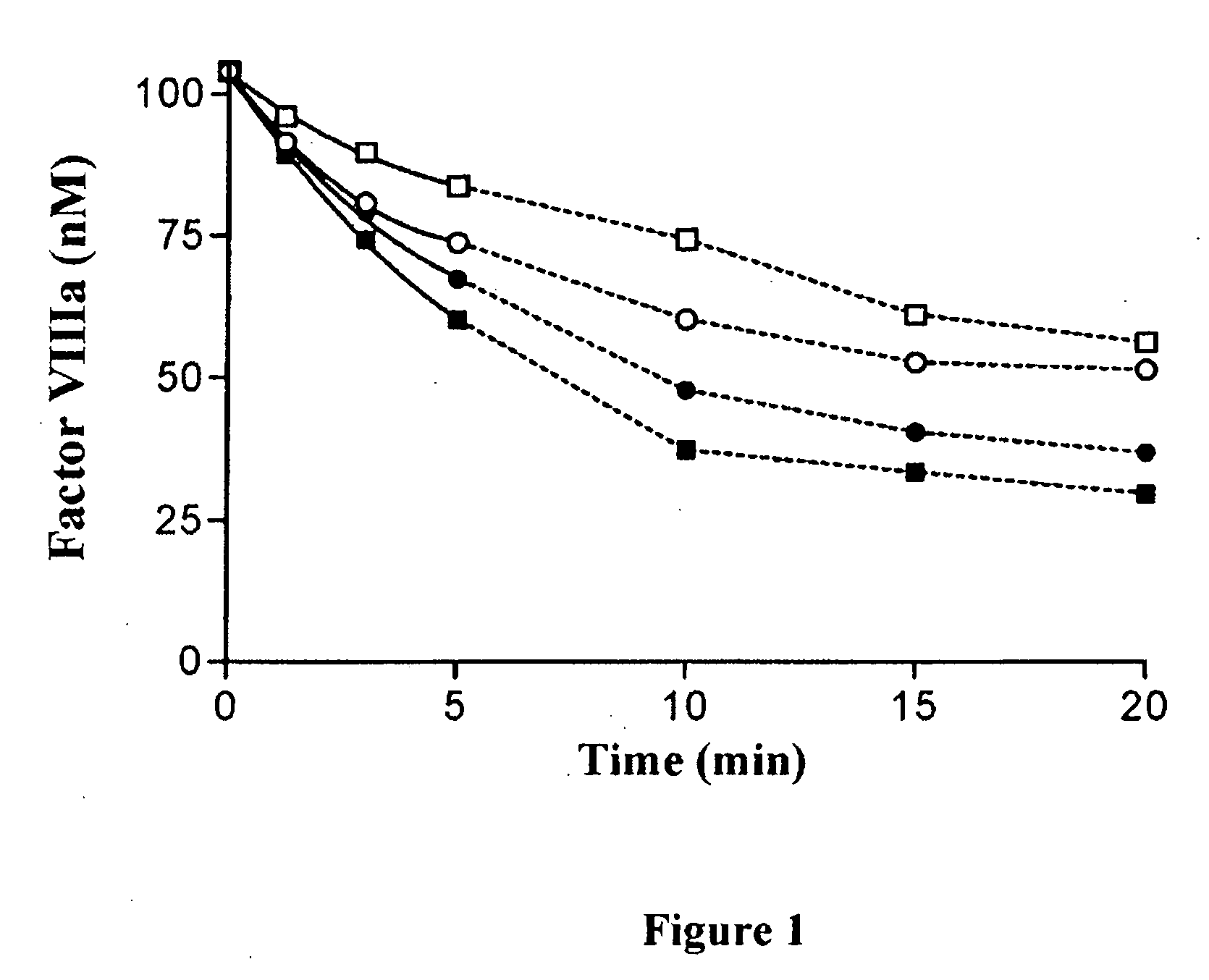
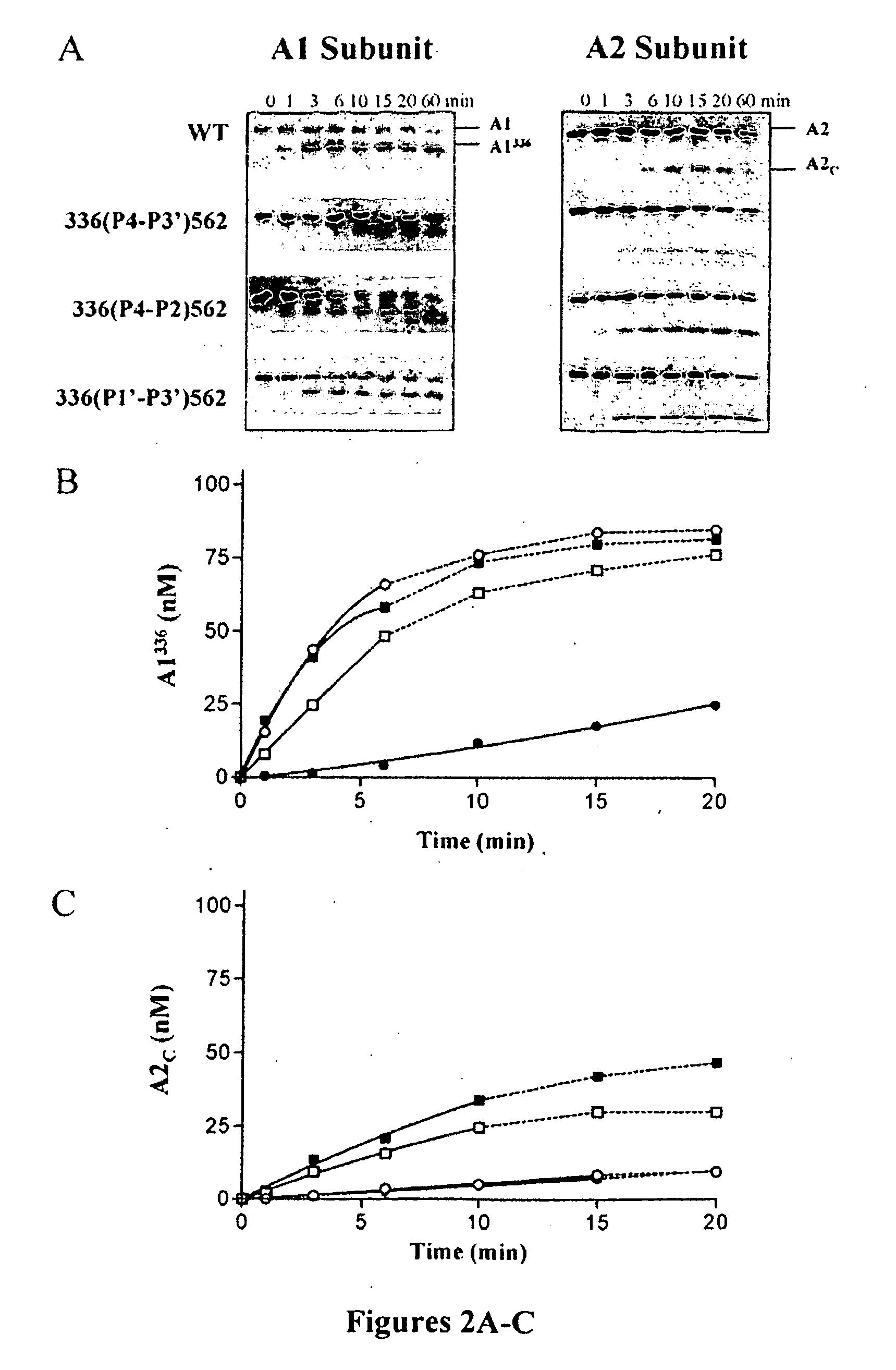
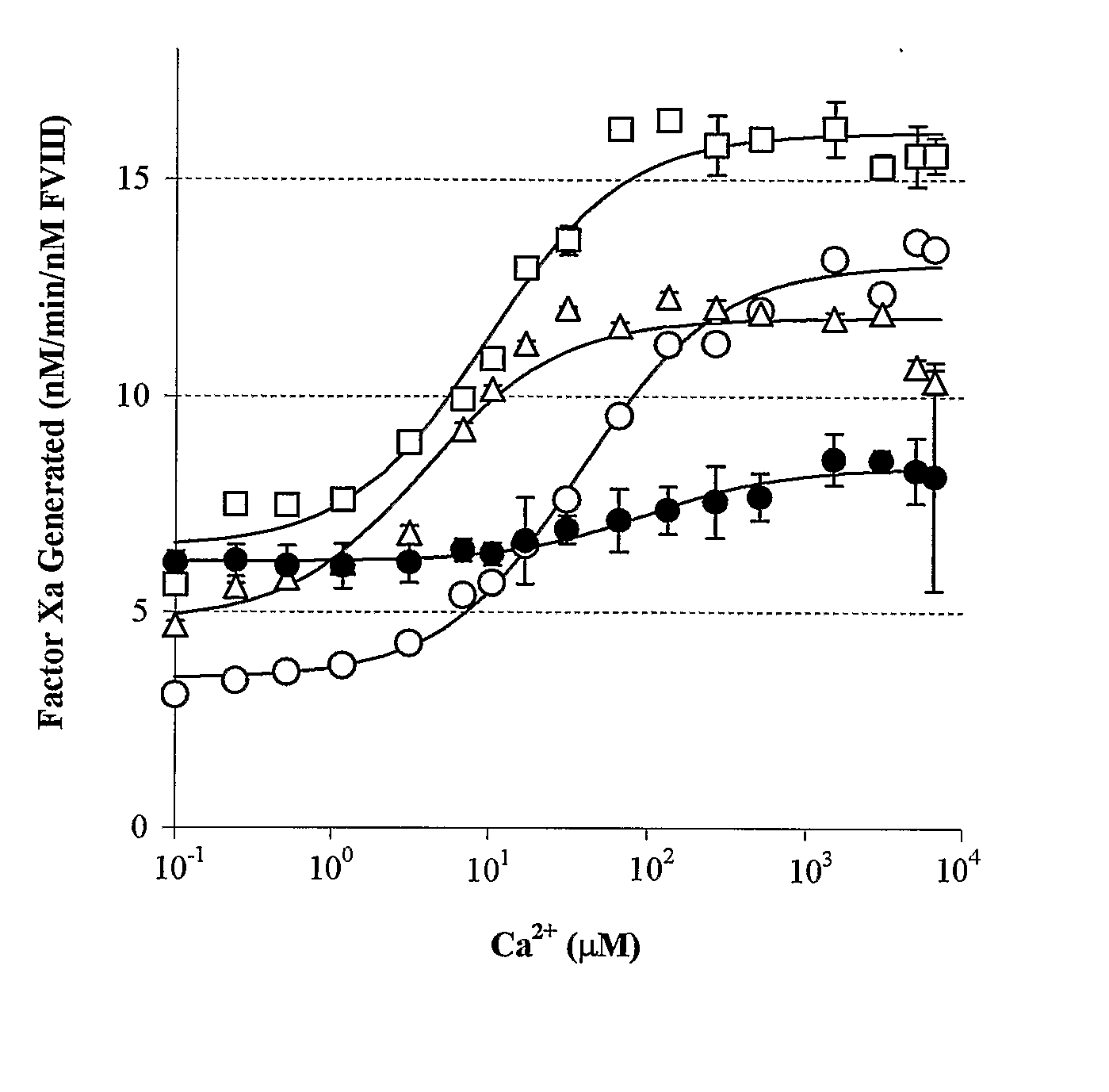
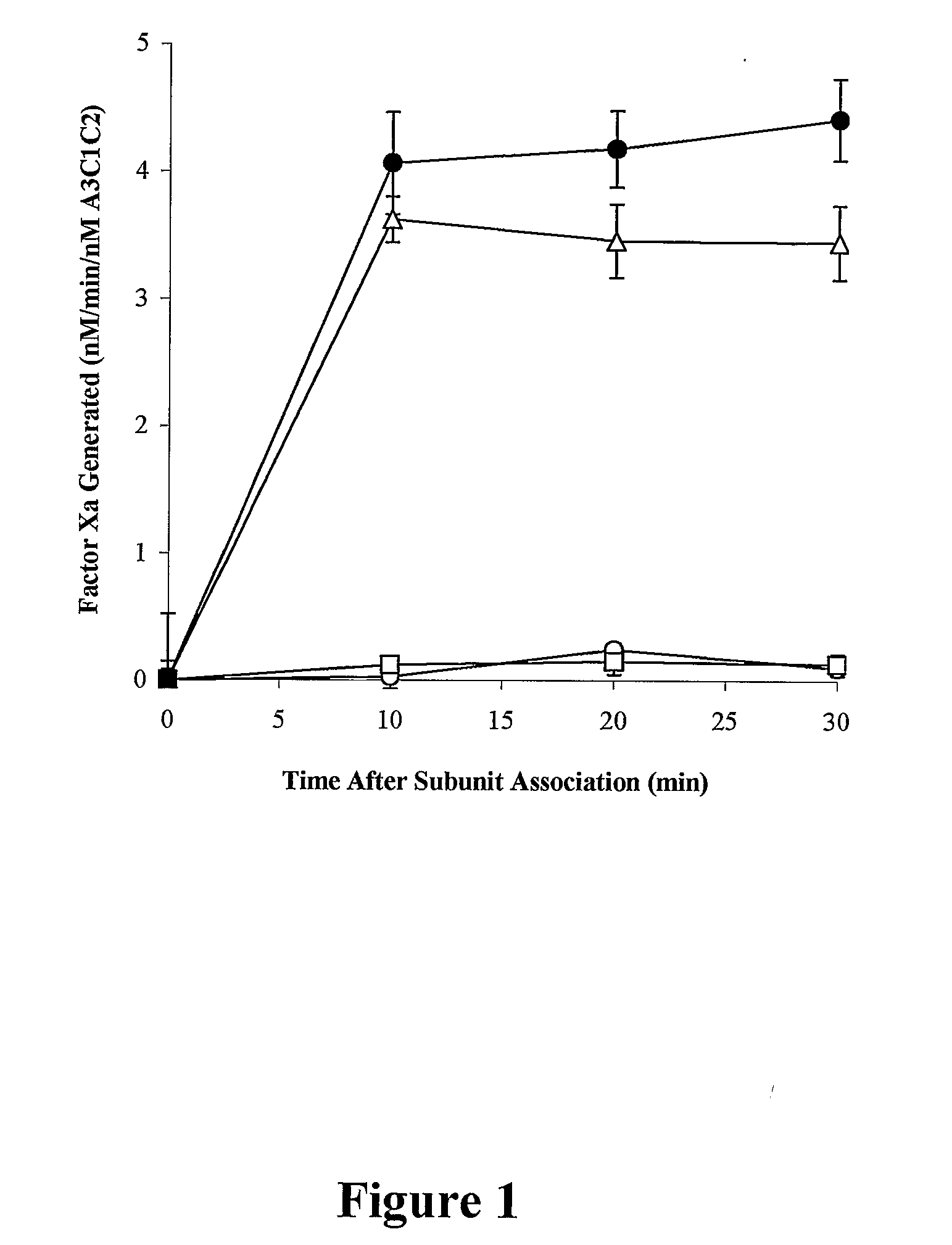
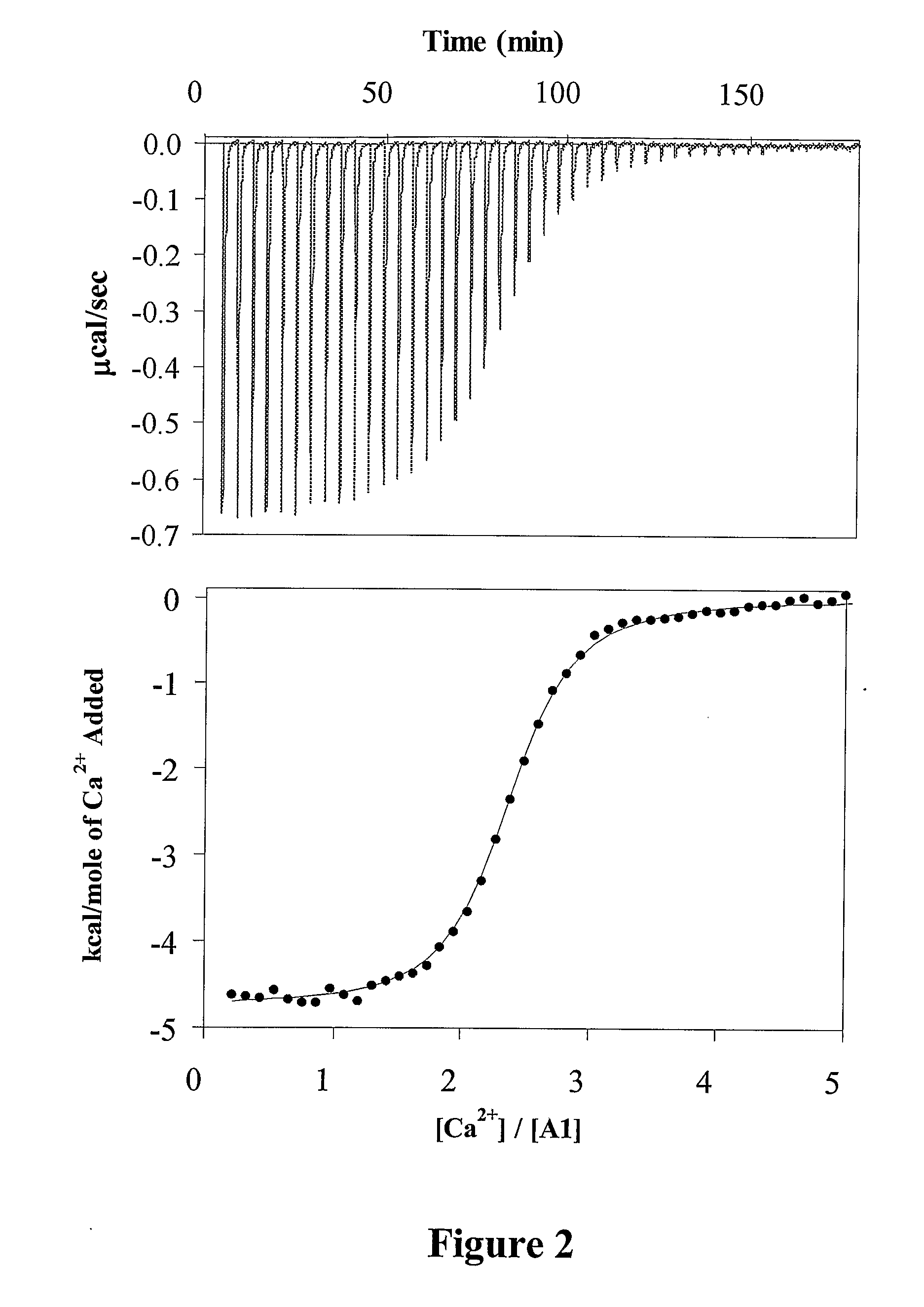
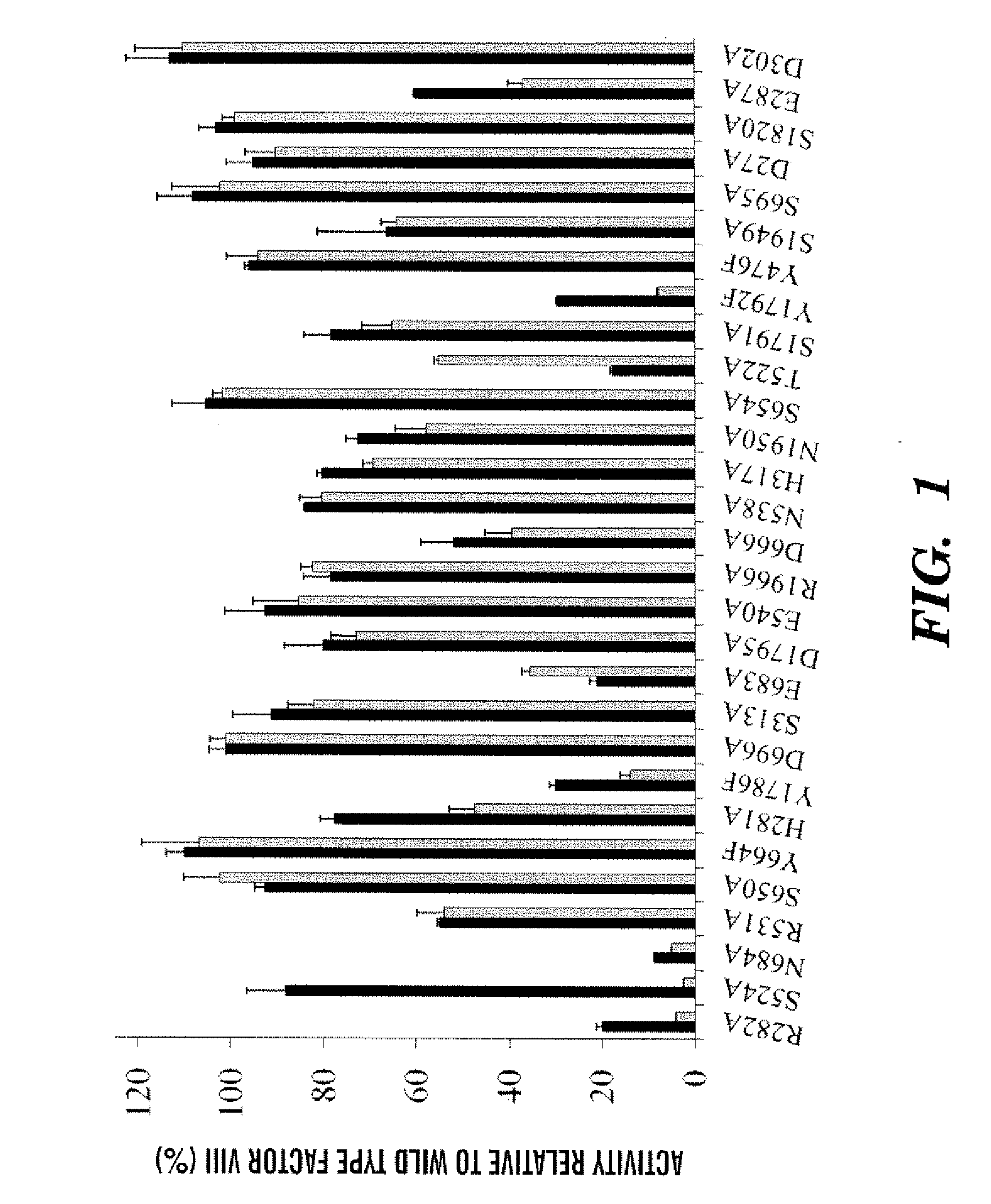
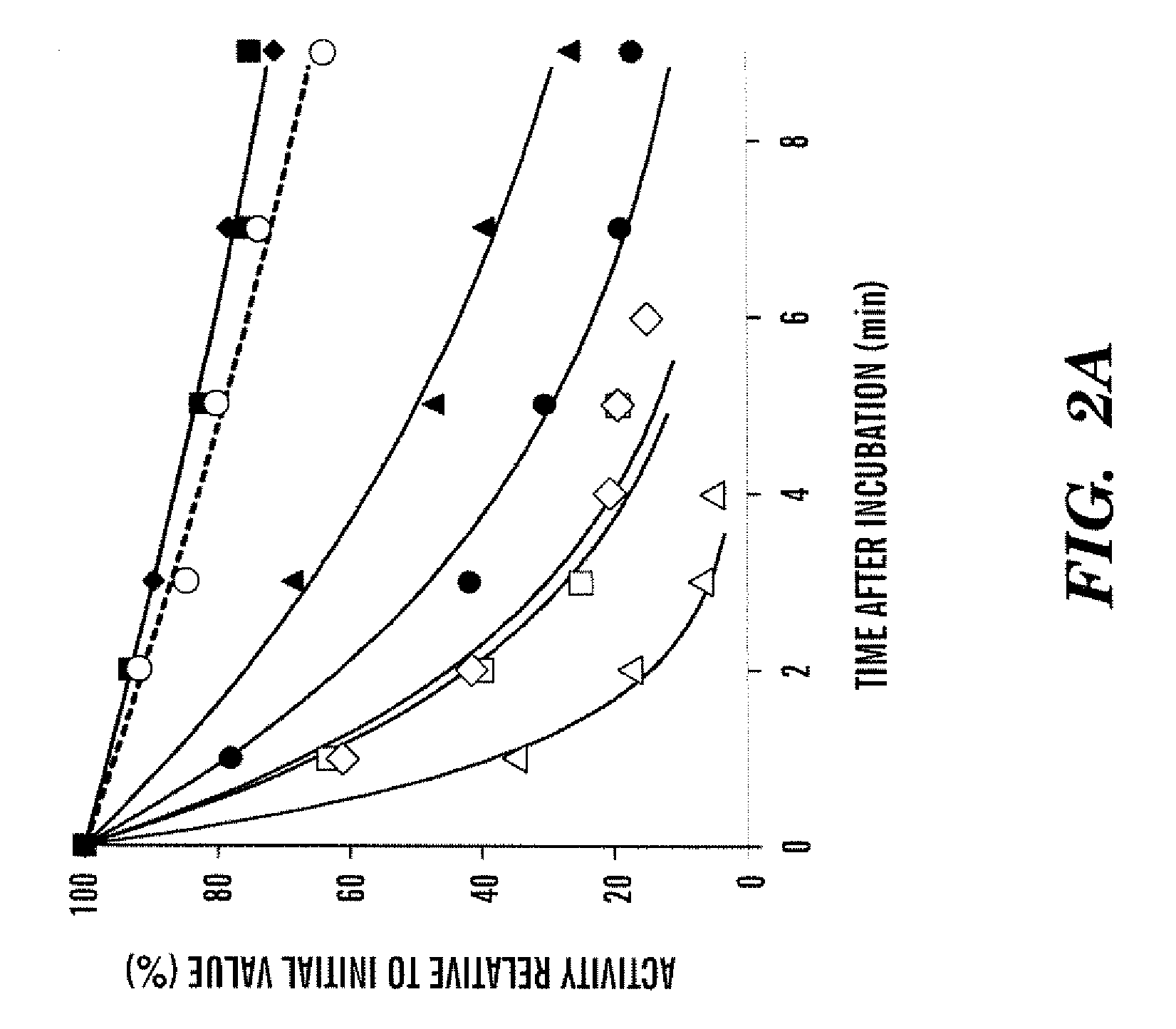
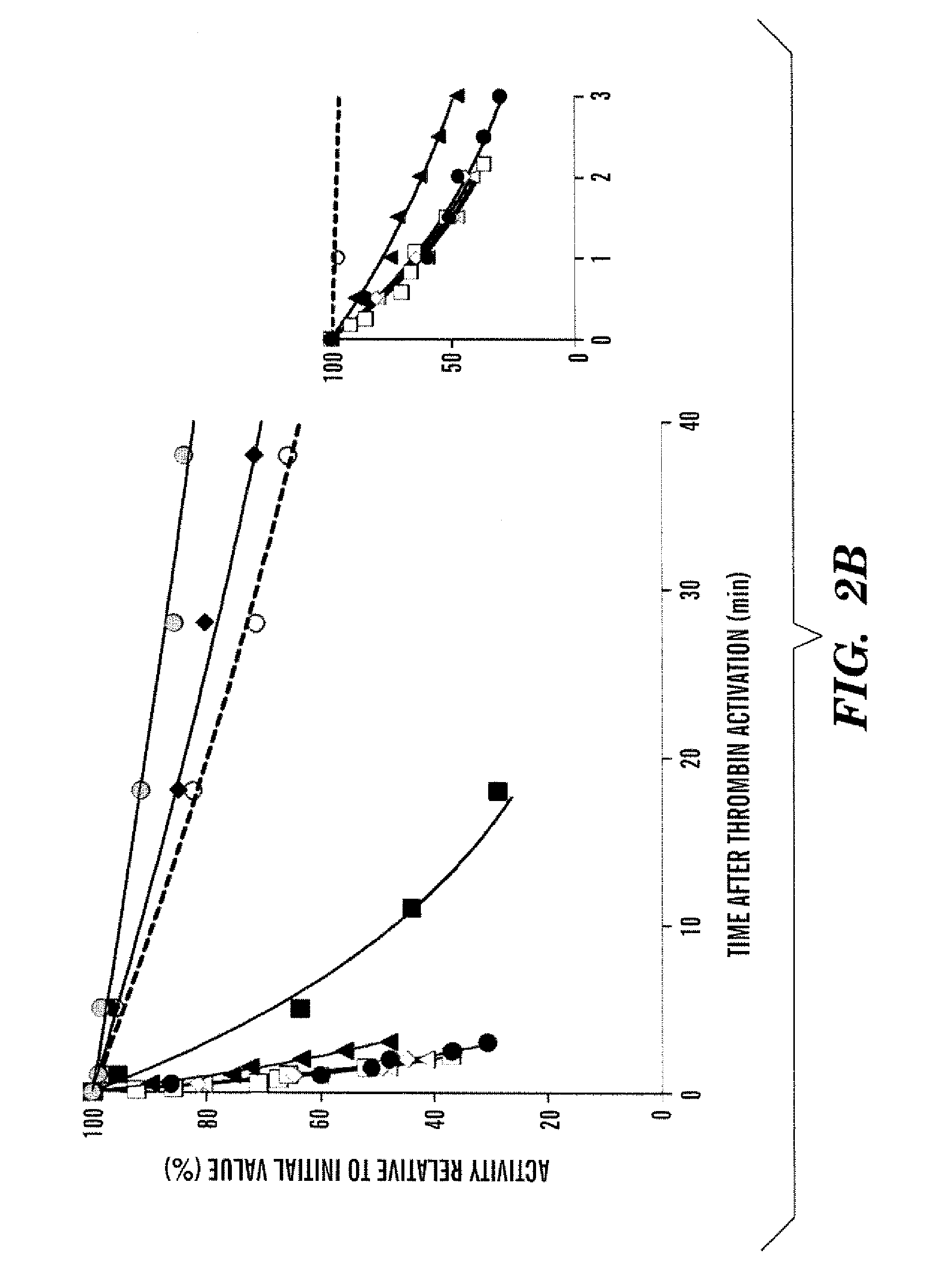
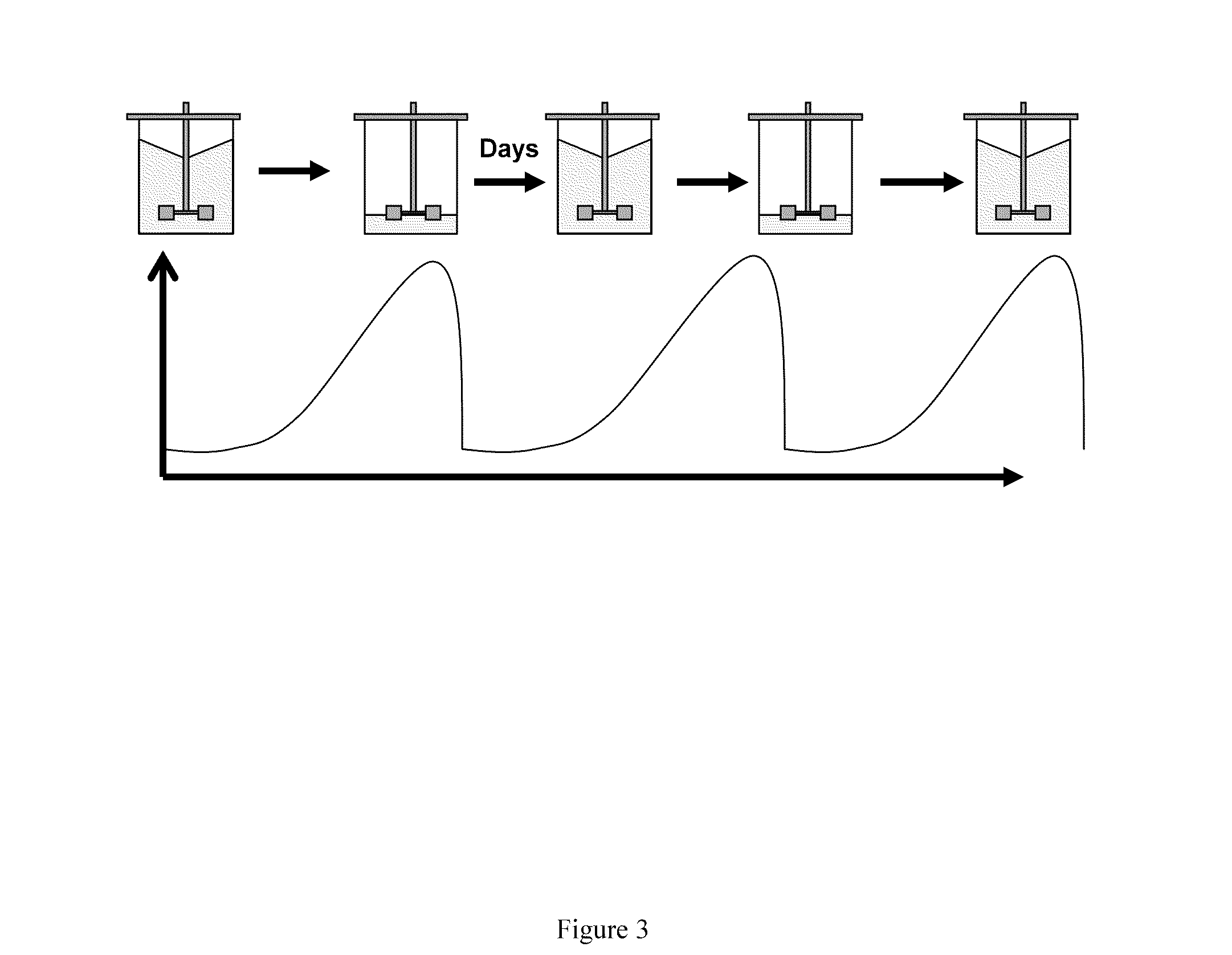
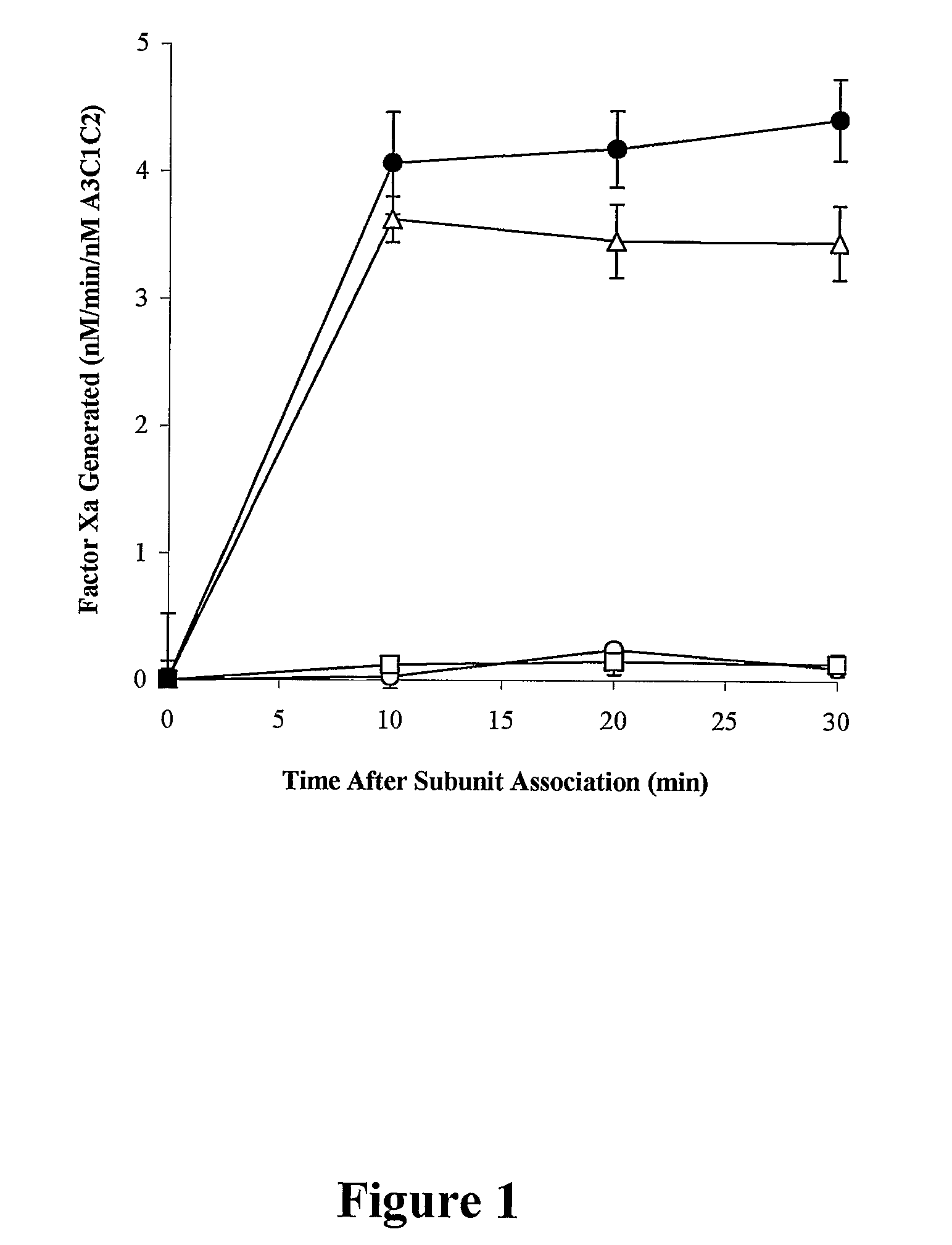
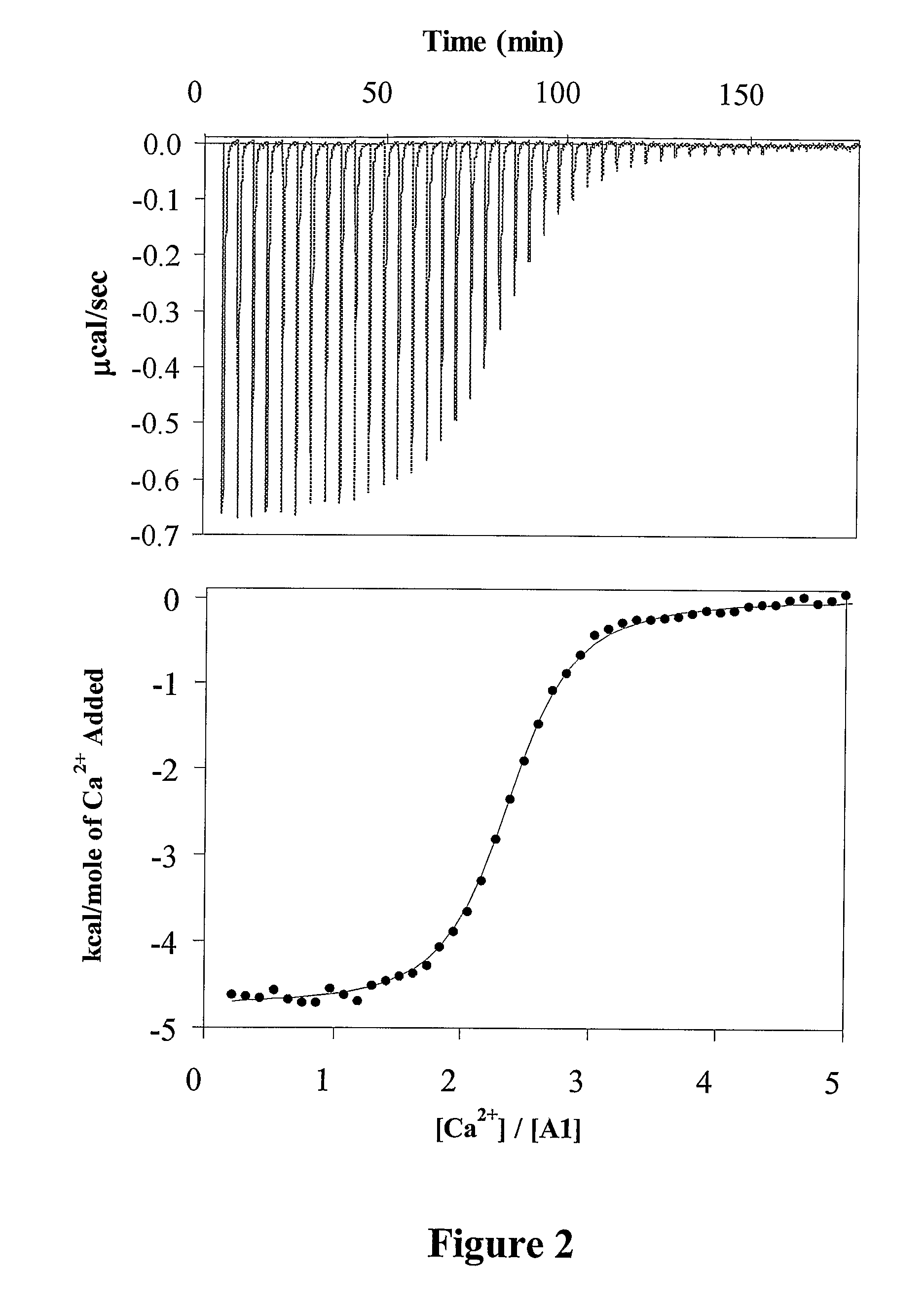
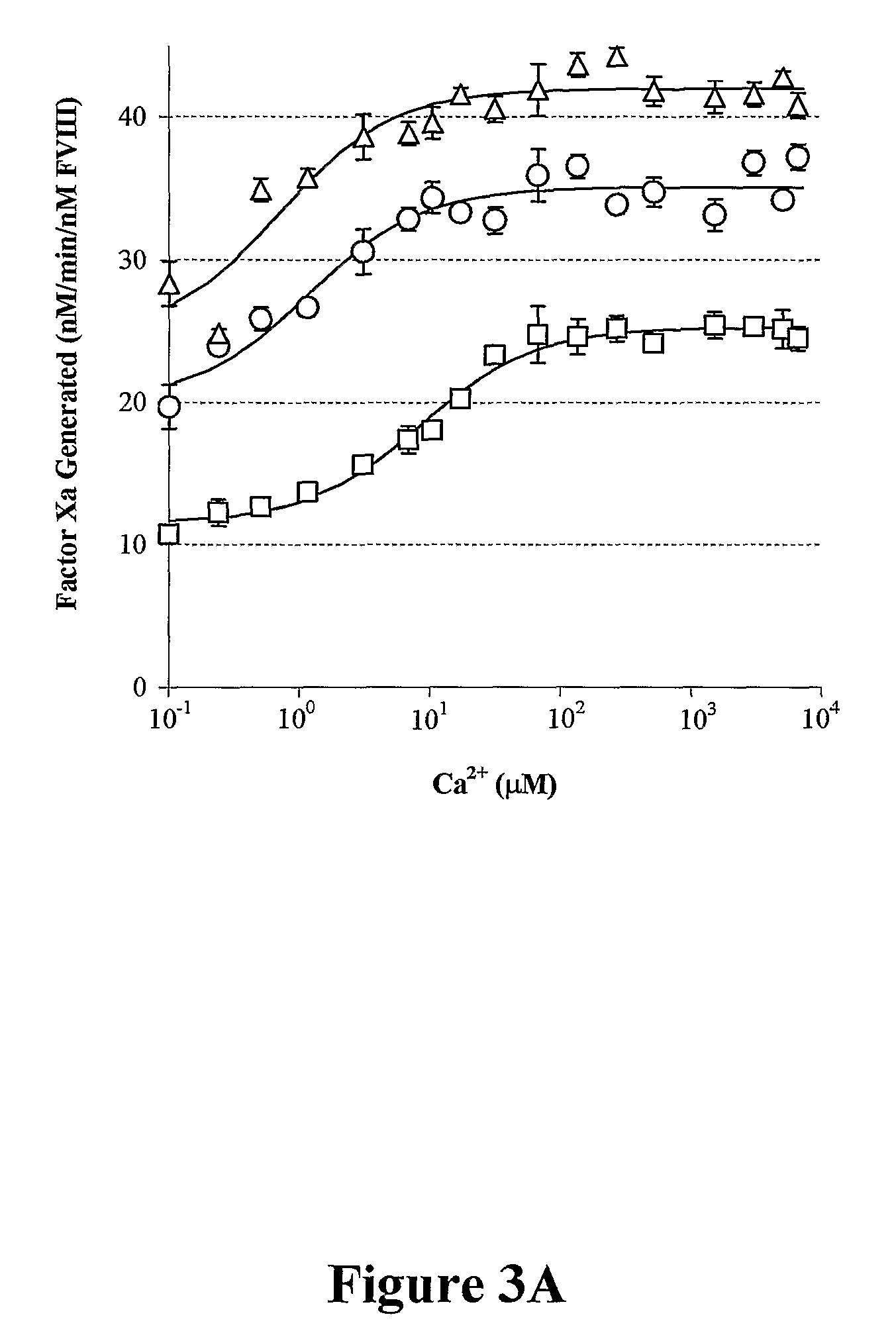
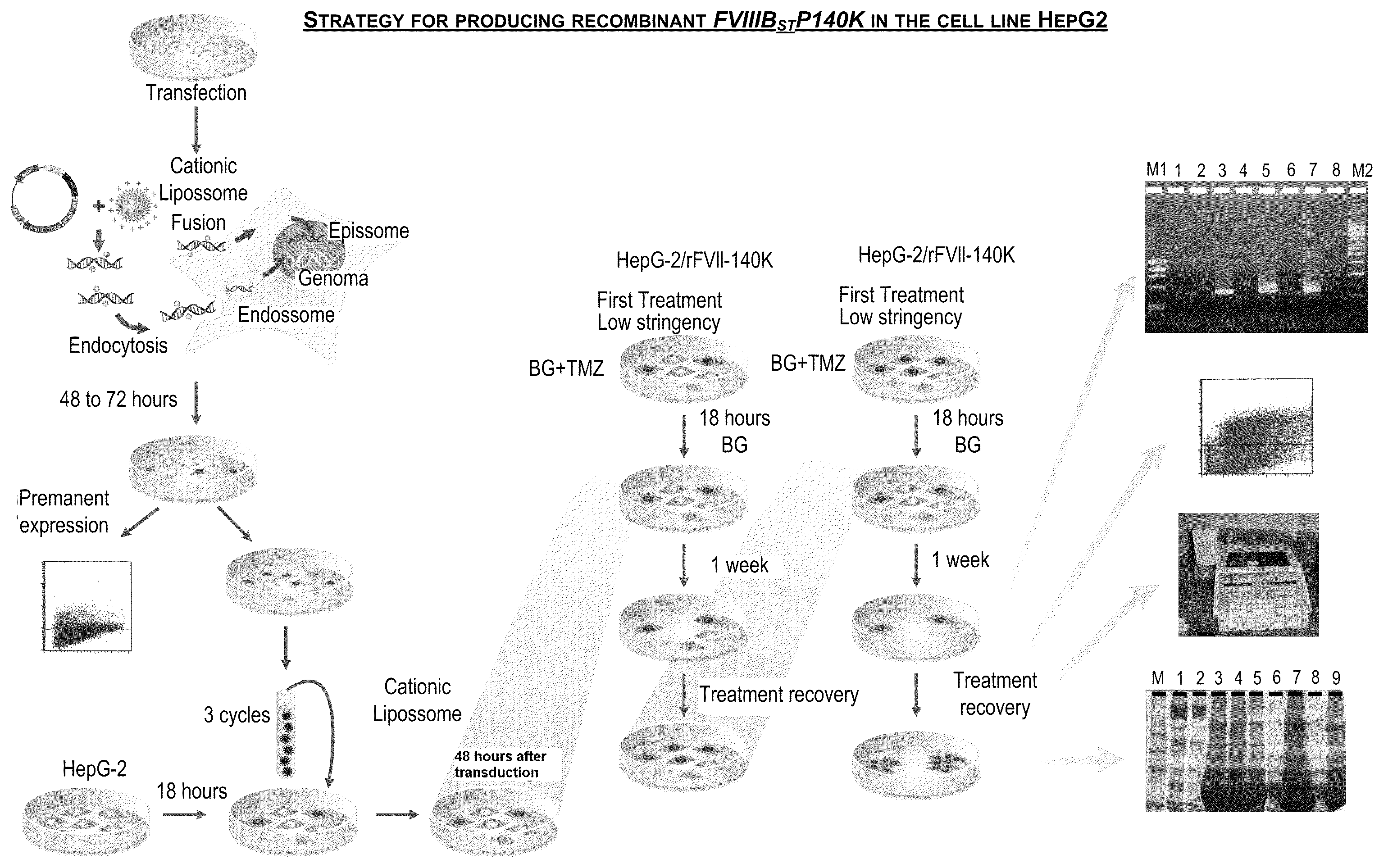
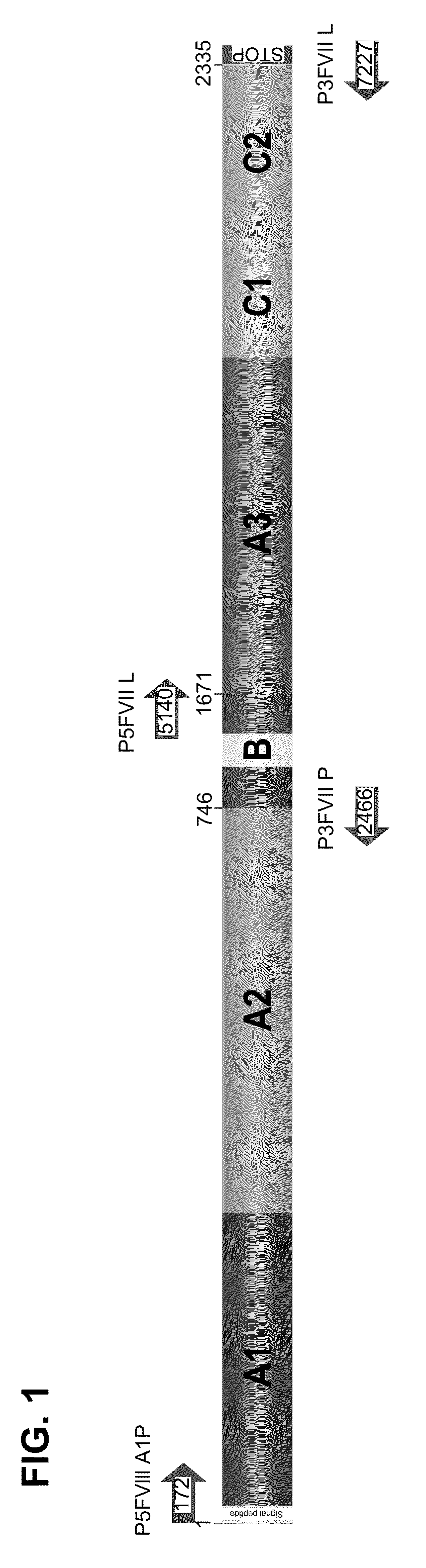
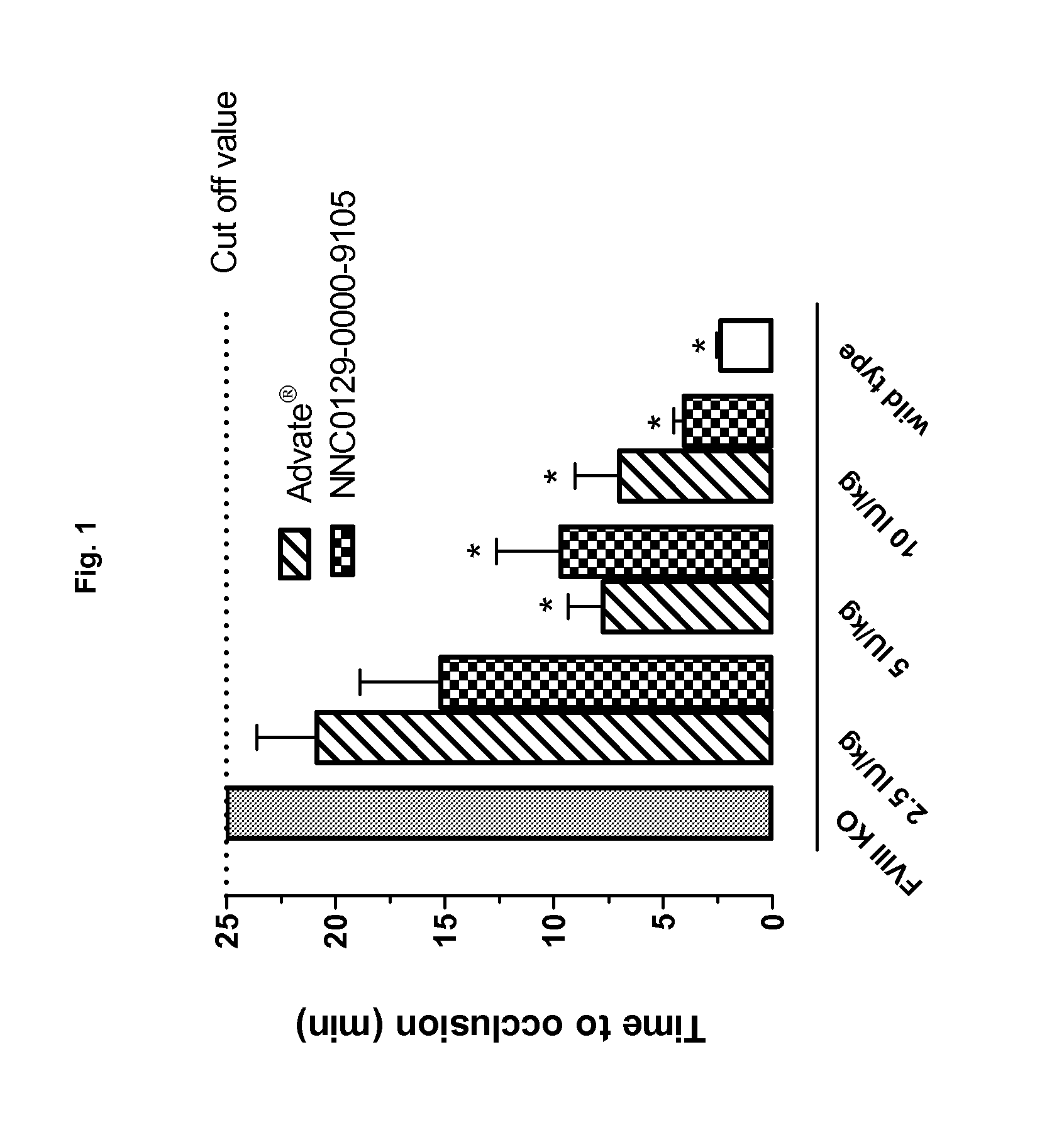
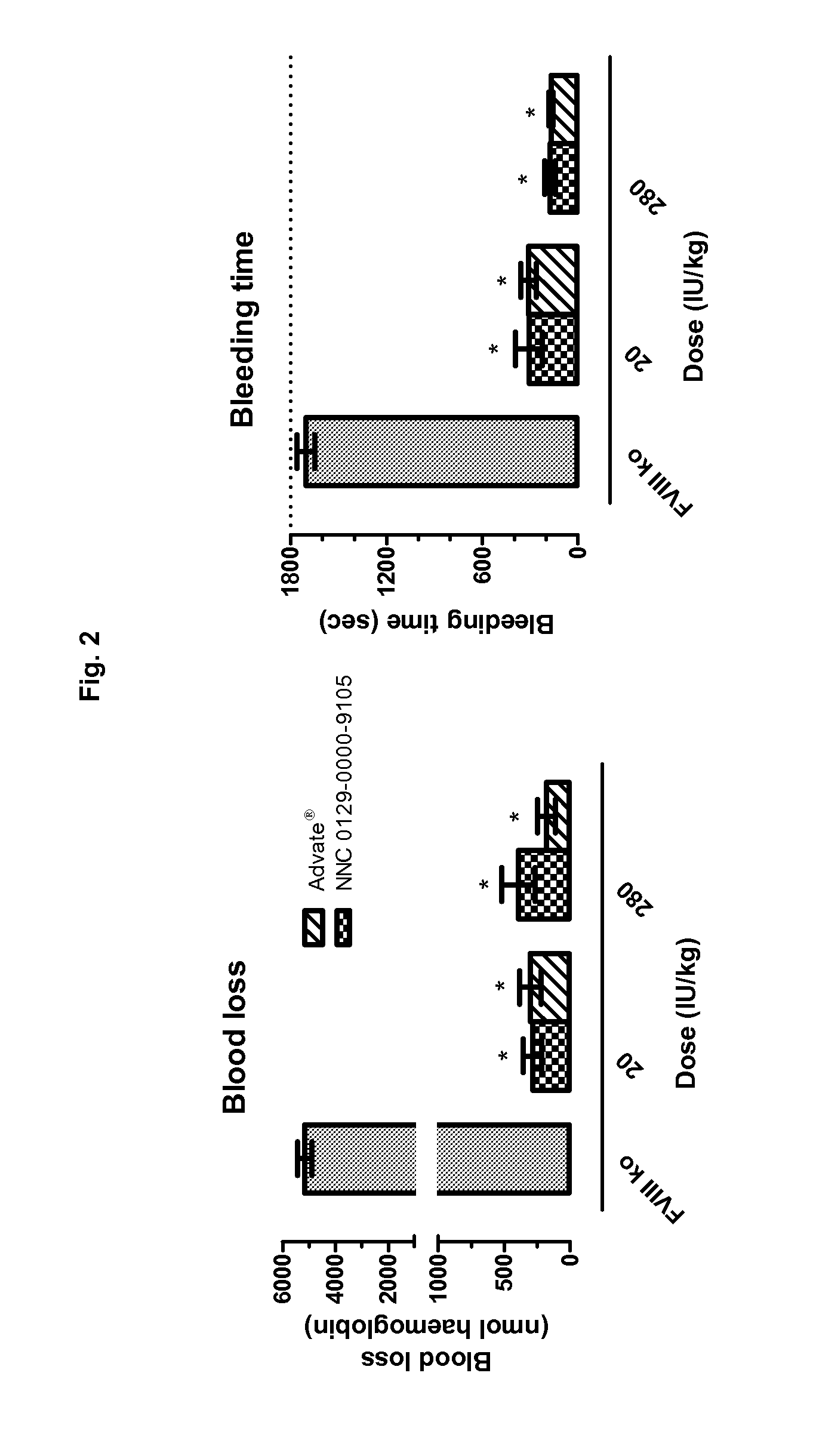
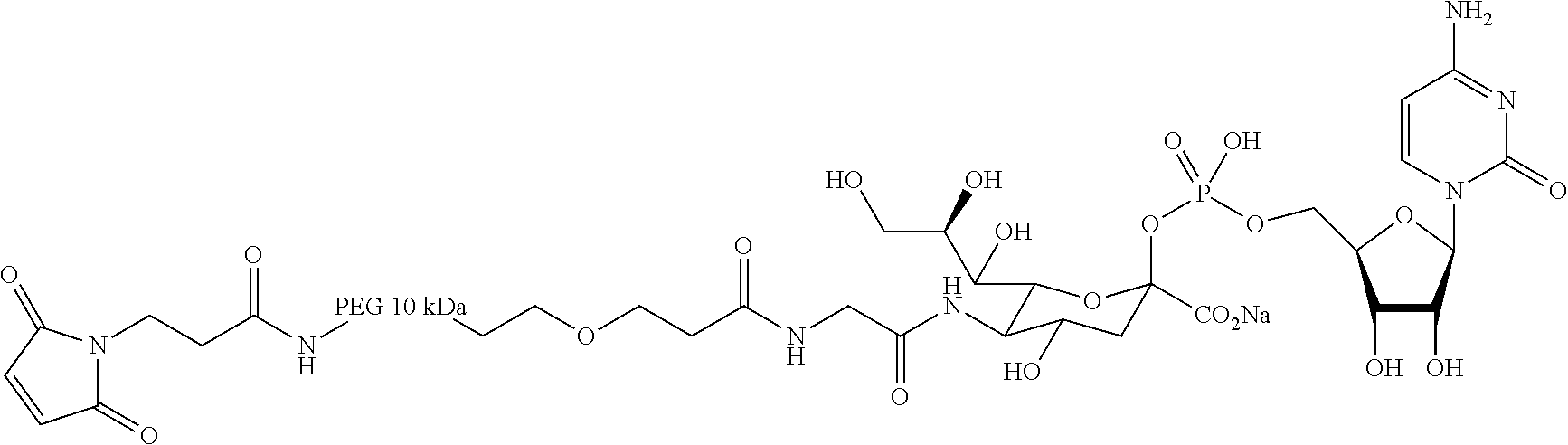
Demannosylated recombinant factor viii [[vii]] for the treatment of patients with haemophilia a
There is provided in accordance with the practice of this invention a demannosylated Factor VIII, the immunogenicity of which is substantially decreased or abolished in Human. The modified factor VIII is disclosed together with the modified amino acid sequence, changed by at least one substitution. The modified factor VIII is useful for hemophiliacs, either to avoid or prevent the action of inhibitory anti-FVIII antibodies.
Owner:LFB BIOTECH +1
Demannosylated recombinant factor VIII for the treatment of patients with haemophilia A
There is provided in accordance with the practice of this invention a demannosylated Factor VIII, the immunogenicity of which is substantially decreased or abolished in Human. The modified factor VIII is disclosed together with the modified amino acid sequence, changed by at least one substitution. The modified factor VIII is useful for hemophiliacs, either to avoid or prevent the action of inhibitory anti-FVIII antibodies.
Owner:LFB BIOTECH +1
Demannosylated recombinant factor viii for the treatment of patients with hemophiila a
Owner:LFB BIOTECH +1
Purification of factor VIII using a mixed-mode or multimodal resin
ActiveUS8399620B2Fast and efficient purificationWithout loss activityFactor VIIMammal material medical ingredientsLoad stepMixed mode
A method for purifying a recombinant protein using a multimodal or mixed mode resin containing ligands which comprise a hydrophobic part and a negatively charged part is described. The invention is advantageous in that it is a single step chromatographic process which does not require adjustment of pH or conductivity during loading step and results in high yield and potency. The process is used for the purification of recombinant compositions of coagulation factor, particularly recombinant Factor VIII.
Owner:NOVO NORDISK AS
Method for the production of recombinant human factor viii
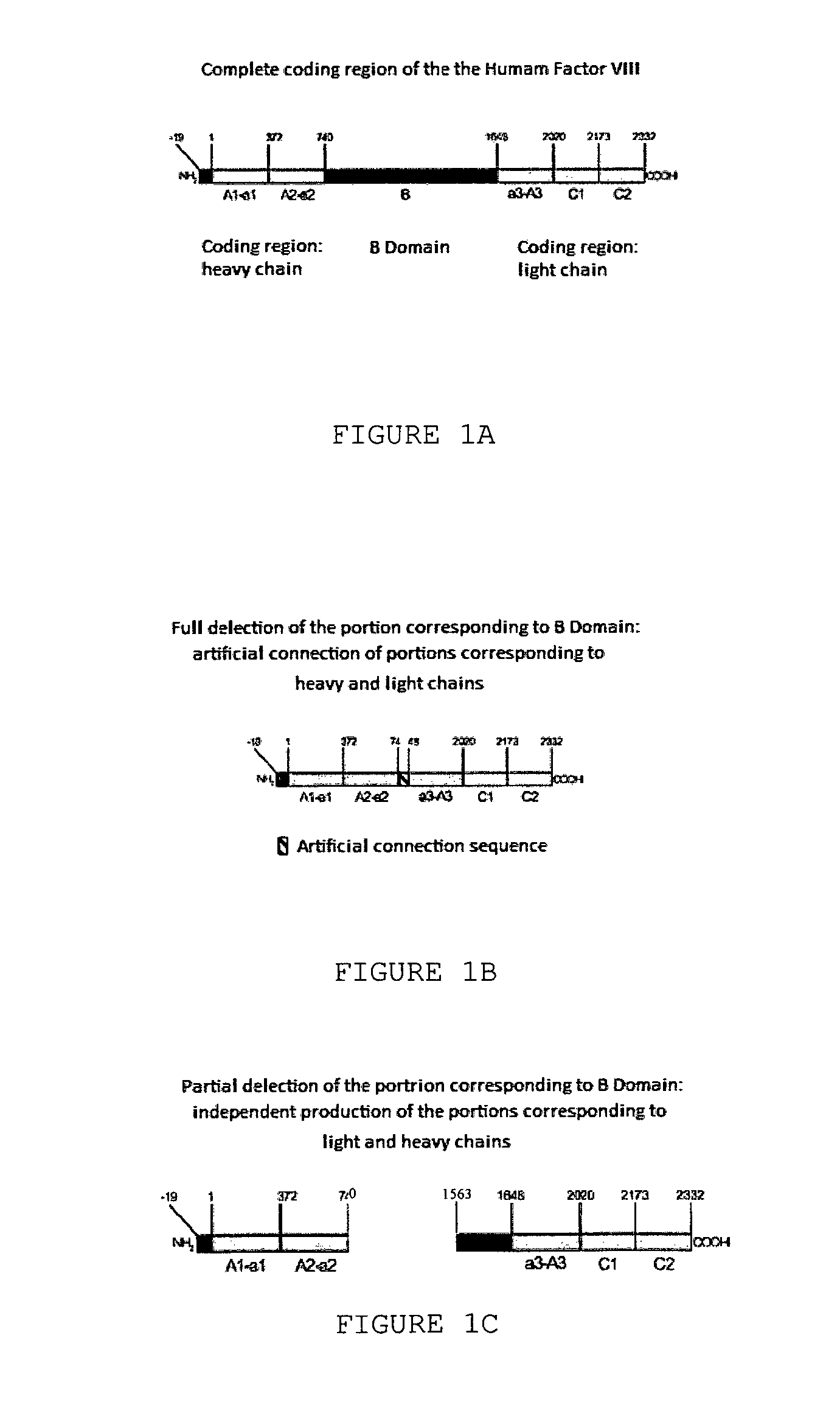
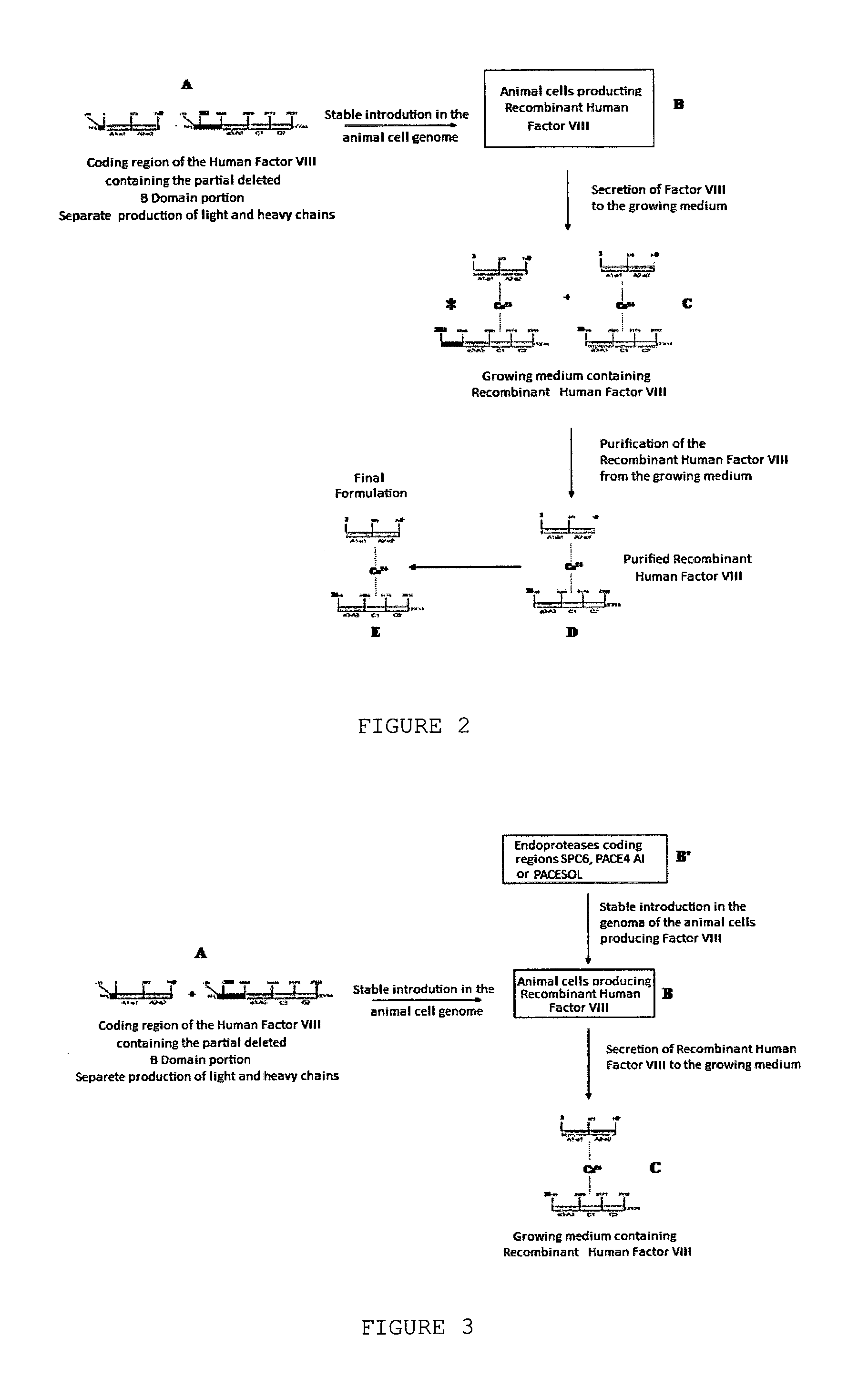
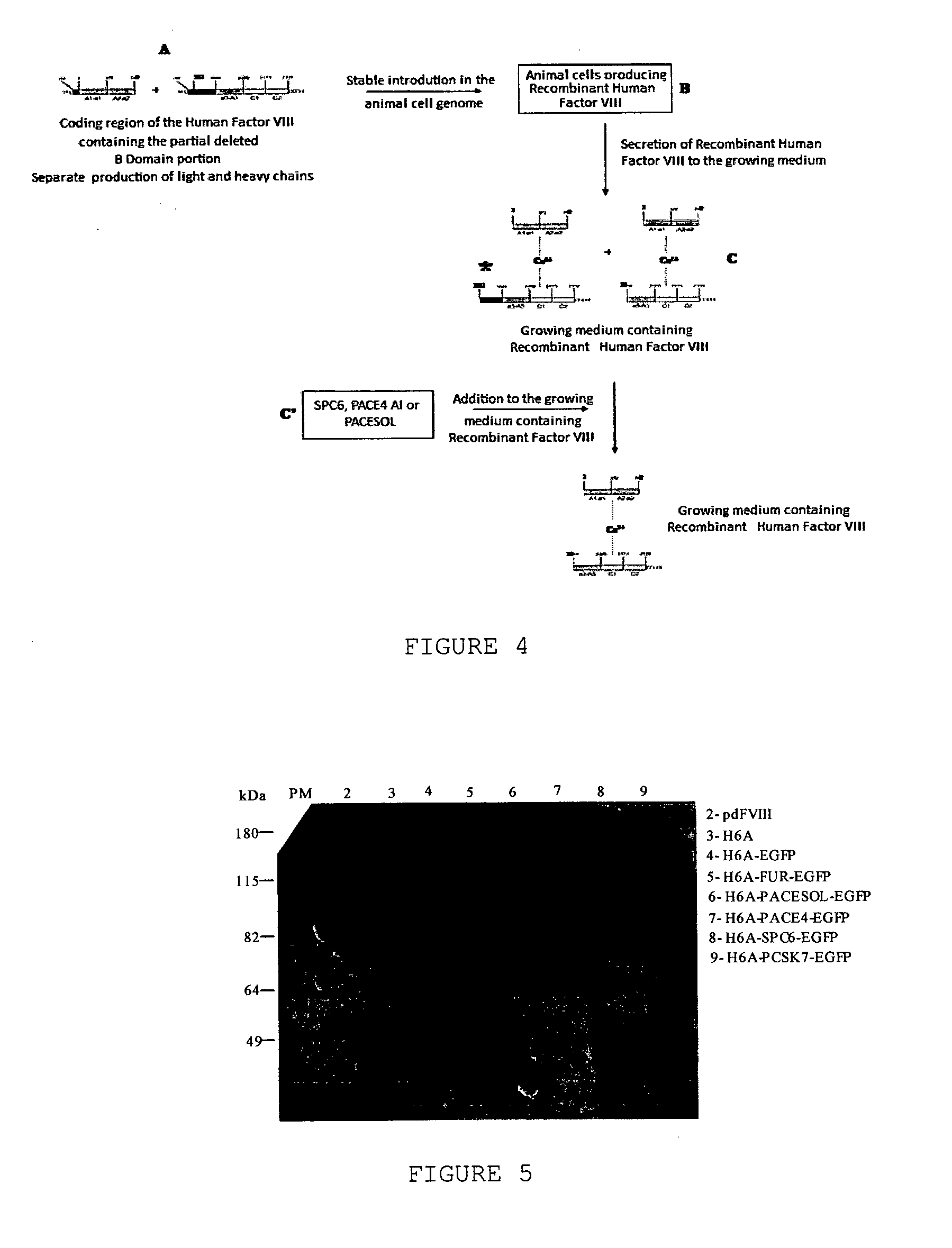
InactiveUS20140051832A1Improve efficiencyCorrect and efficient foldFactor VIIMammal material medical ingredientsFactor iiProteolysis
The object of the present invention is to provide methods for the production of recombinant human Factor VIII, employing specific endoproteases, thus assuring full proteolytic processing of said factor even during its biosynthesis, consequently avoiding additional purification steps. Other objects of the present invention are the recombinant human Factor VIII as obtained by said methods, pharmaceutical compositions, related uses and therapeutic methods.
Owner:UNIV DE SAO PAULO
Recombinantly produced human factor viii and ix
ActiveUS20110262424A1Improve bindingImprove functional propertiesFactor VIIPeptide/protein ingredientsN-Glycolylneuraminic acidGlycosylation
A recombinant human factor VIII or IX protein having a human glycosylation pattern but the protein is devoid of N-glycolylneuraminic acid and / or the carbohydrate group Galα-3Gal.
Owner:OCTAPHARMA
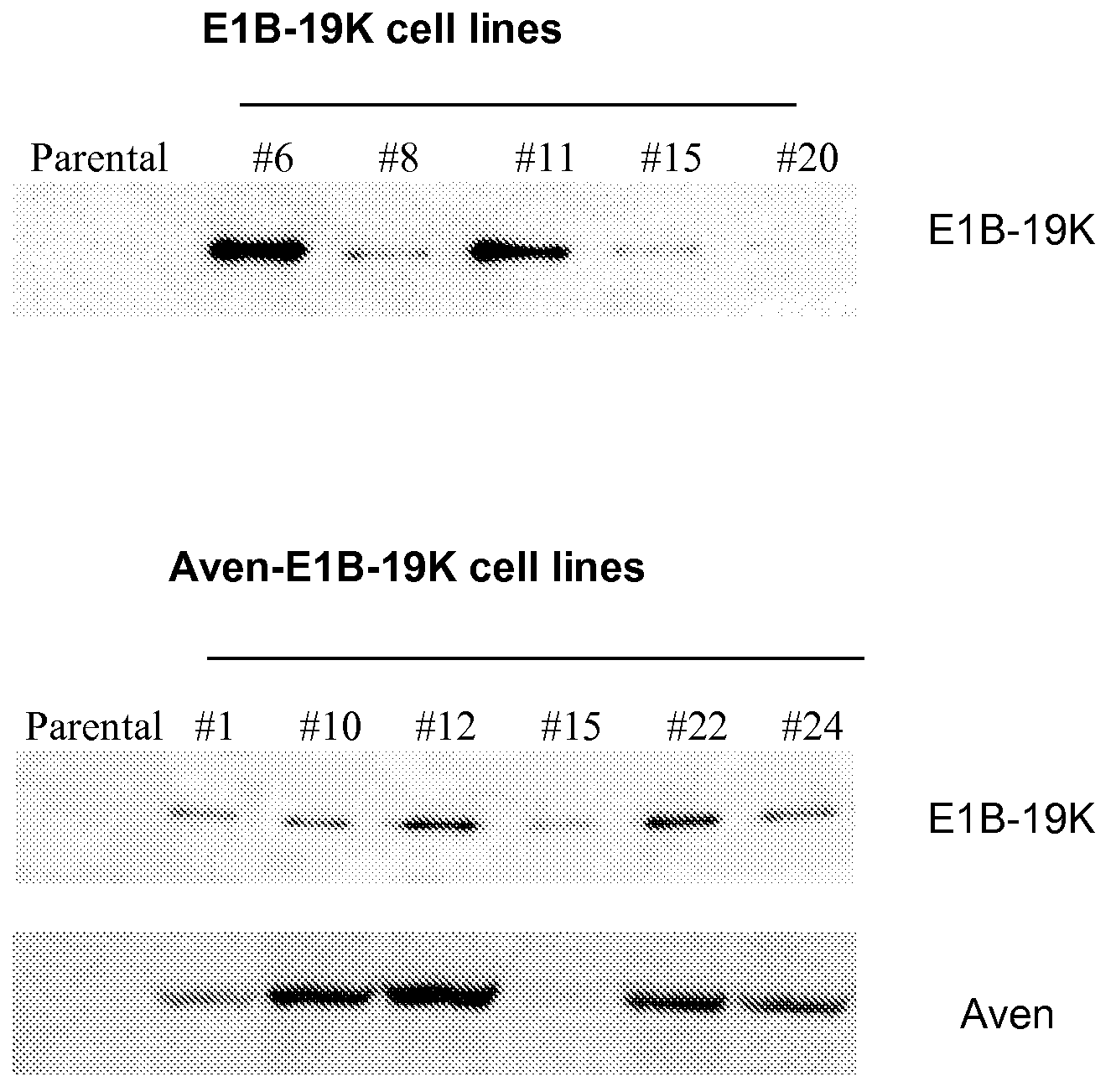
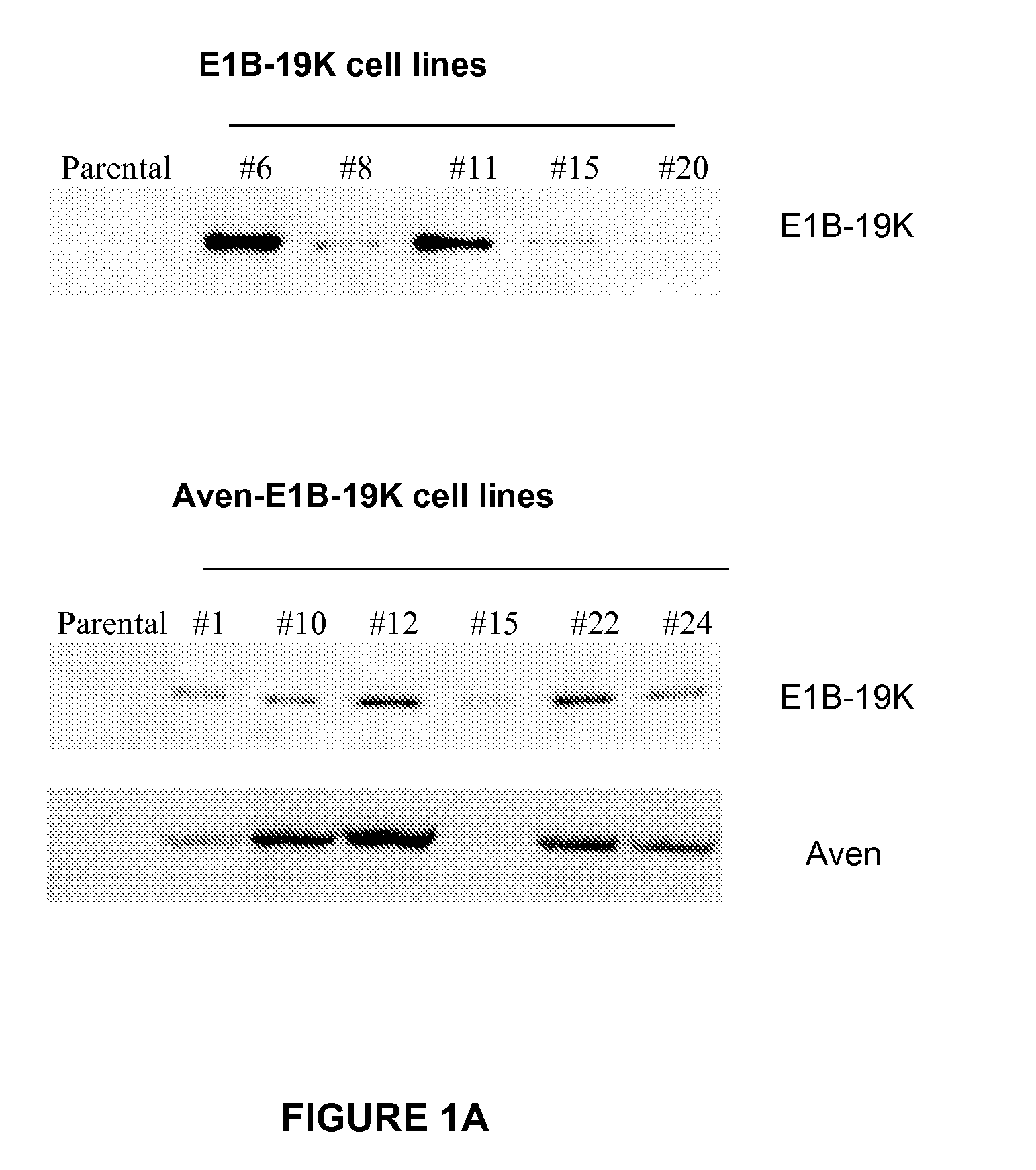
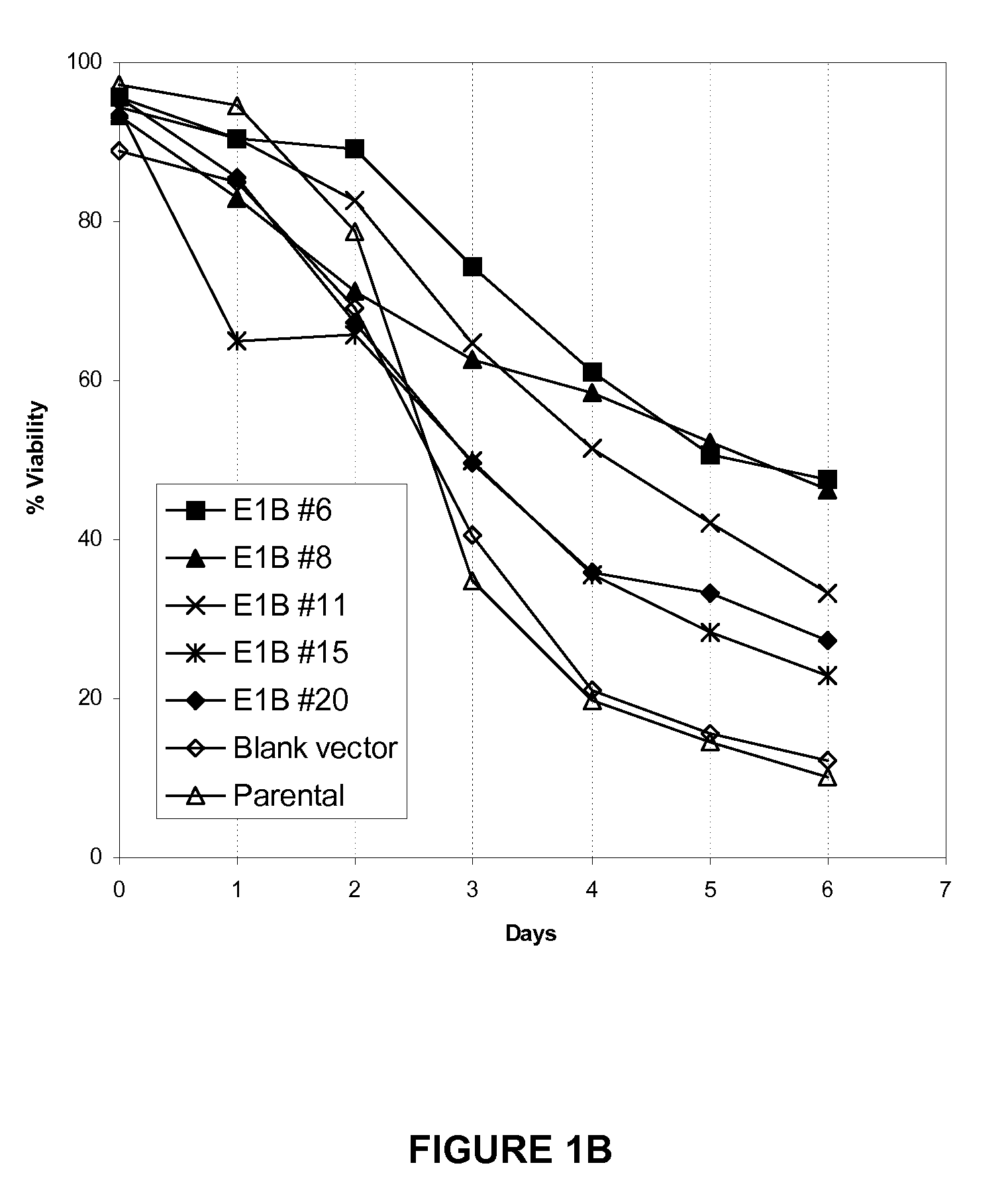
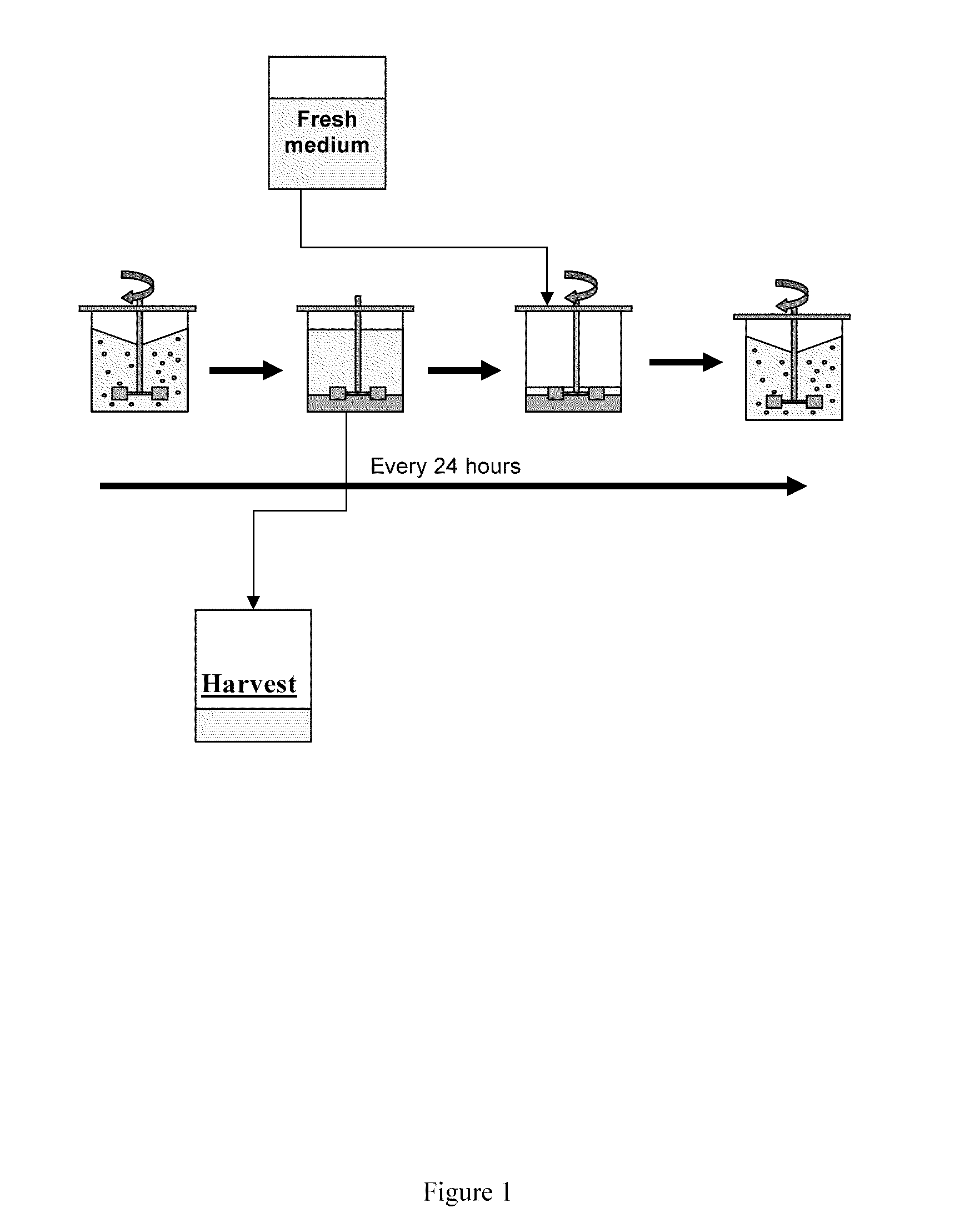
Application of Anti-Apoptotic Gene Expression in Mammalian Cells for Perfusion Culture
InactiveUS20100167396A1Preventing or delaying programmed cell deathCell death in cell is prevented and delayedAnimal cellsPeptide/protein ingredientsPerfusion CultureAnti apoptotic genes
The present invention relates to preventing or delaying programmed cell death by expressing one or more anti-apoptotic polypeptides in a cell expressing recombinant Factor VIII such that programmed cell death in the cell is prevented or delayed. The present invention also relates to increasing production of recombinant Factor VIII by expressing one or more anti-apoptotic polypeptides in a cell such that production of recombinant Factor VIII by the cell is increased. Recombinant cells useful for producing Factor VIII.
Owner:BAYER HEALTHCARE LLC
Mutant factor viii compositions and methods
In one aspect, present invention provides a recombinant mutant human factor VIII having increased expression and / or secretion as compared to wild-type factor VIII. In certain embodiments, the recombinant factor VIII includes one or more amino acid substitution(s) selected from the group consisting of I86, Y105, A108, D115, Q117, F129, G132, H134, M147 and L152. In other aspects, the present invention provides FVIII encoding nucleic acids, FVIII-expression vectors, as well as methods of using the modified FVIII genes in the treatment of FVIII deficiencies, such as hemophilia A.
Owner:XIAO WEIDONG
Method of purifying factor VII and/or factor VIIa
The present invention relates generally to a method of purifying Factor VII and / or Factor VIIa from a solution containing either or both proteins, the method comprising: diluting the solution containing Factor VII and / or Factor VIIa in a loading buffer; adding the diluted solution from step (i) to a multi-modal anion exchange resinunder conditions selected such that Factor VII and / or Factor VIIa is bound to the resin; optionally washing the resin with a wash buffer; adding an elution buffer to the resin under conditions selected such that Factor VII and / or Factor VIIa is eluted from the resin; and recovering the eluted recombinant Factor VII and / or Factor VIIa; wherein the loading buffer, the optional wash buffer and the elution buffer each comprise about 35 mM or less of calcium ions.
Owner:CSL LTD
Recombinant factor VIII having enhanced stability following mutation at the A1-C2 domain interface
InactiveUS8637448B2Improve stabilityExtended half-lifeFactor VIIBacteriaPharmaceutical drugHemophilias
Owner:UNIVERSITY OF ROCHESTER
De-immunized factor viii molecule and pharmaceutical compositions comprising the same
The present invention relates to the field of therapeutic proteins, in particular, to recombinant coagulation factors. It provides a recombinant Factor VIII (FVIII) protein comprising specific point mutations at defined positions, which serve to reduce the immunogenicity of said FVIII protein, wherein the Factor VIII protein substantially retains its coagulant activity. The invention further provides nucleic acids encoding said de-immunized protein, cell lines and methods of recombinant preparation as well as pharmaceutical compositions comprising the recombinant FVIII of the invention, whichare advantageous for use in treatment of patients with Hemophilia A, particularly those who have not yet been treated with a FVIII product. Additionally, it can be a safe alternative for previously treated patients and even for patients who have developed an immune-response to FVIII, e.g., for immune-tolerance-induction therapy (ITI / ITT) or rescue ITI. The invention also provides an assay for determining immunogenicity of a protein.
Owner:BIOTEST SERUM INST GMBH
Recombinant factor C from Tachypleus tridentatus expressed by insect cells
The invention relates to the field of bioengineering, in particular to a method for recombining and expressing a gene of a factor C from Tachypleus tridentatus in insect cells so as to detect and remove endotoxin. The method comprises the steps of preparing a recombinant rhabdovirus of the gene of the factor C from Tachypleus tridentatus, transfecting the insect cells, detecting the expression of the recombinant factor C from Tachypleus tridentatus in the insect cells, and the like. The recombinant factor C from Tachypleus tridentatus, prepared by the method, provides a precondition for researching the binding activity of endotoxin of the factor C, and also lays a foundation for the application of the factor C in detection and removal of the endotoxin.
Owner:SHANGHAI BAISHENG BIOTECH CO LTD
Modified recombinant factor viii and von willebrand factor and methods of use
Owner:BAXTER INT INC +1
Treatment of gastrointestinal bleeding in patients with severe von willebrand disease by administration of recombinant vwf
ActiveUS20190091299A1High specific activityPeptide/protein ingredientsBlood disorderFactor VIII vWFVon willebrand
The present invention relates to a method for treating gastrointestinal bleeding in a subject with severe von Willebrand Disease comprising administering to the subject at least one dose of recombinant von Willebrand Factor (rVWF) ranging from about 40 IU / kg to about 100 IU / kg, wherein the first dose further comprises recombinant Factor VIII (rFVIII).
Owner:TAKEDA PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
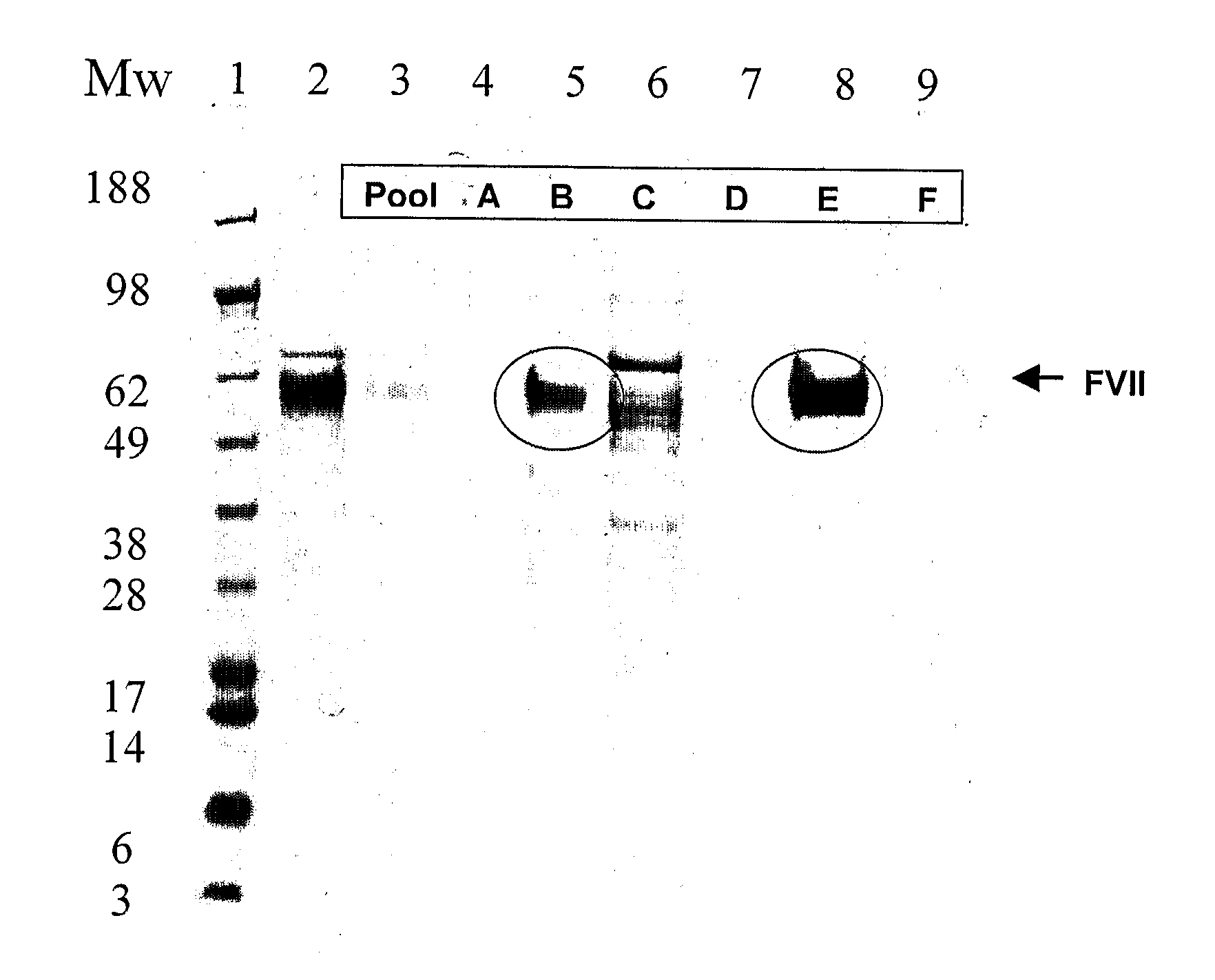
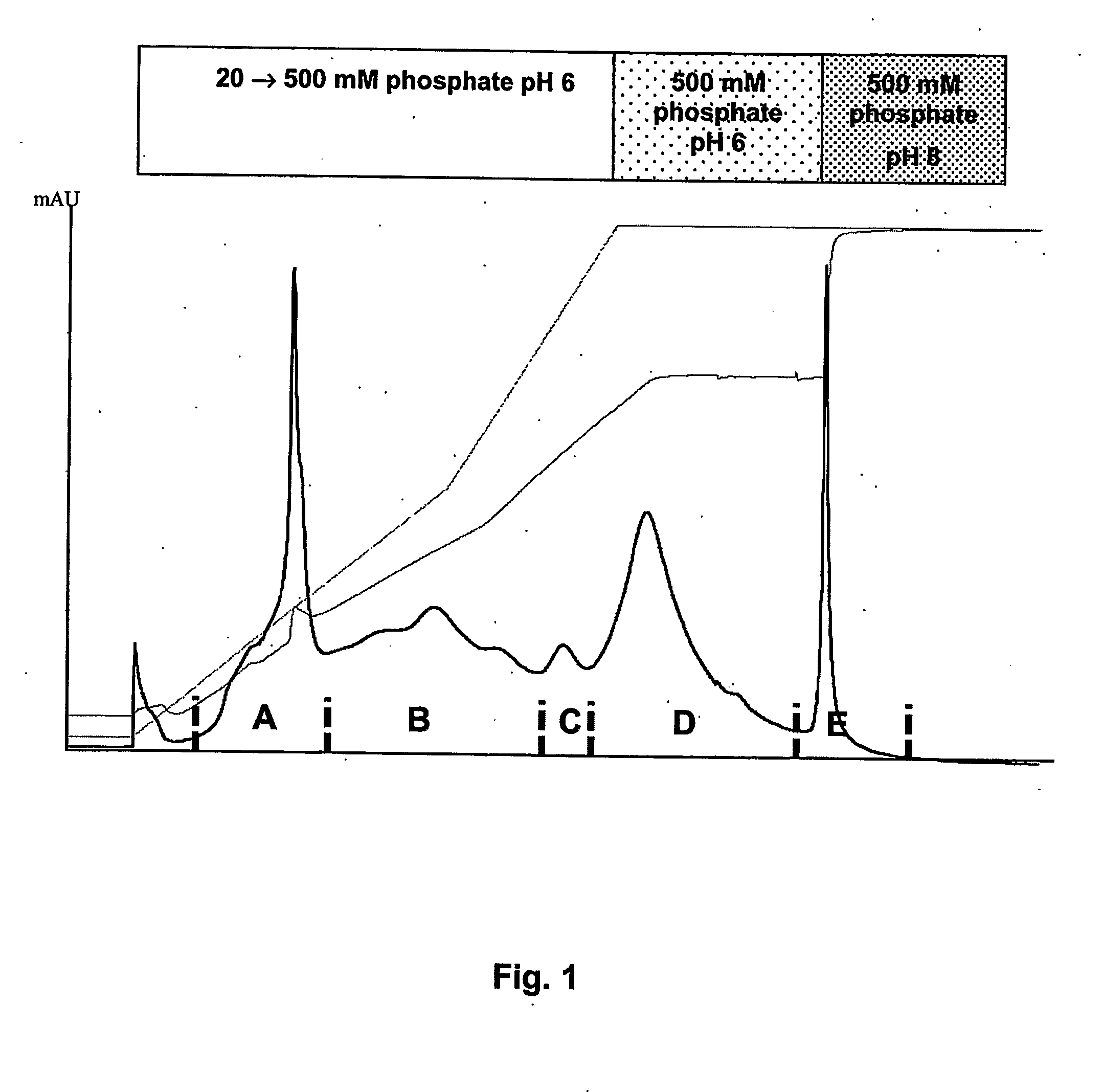
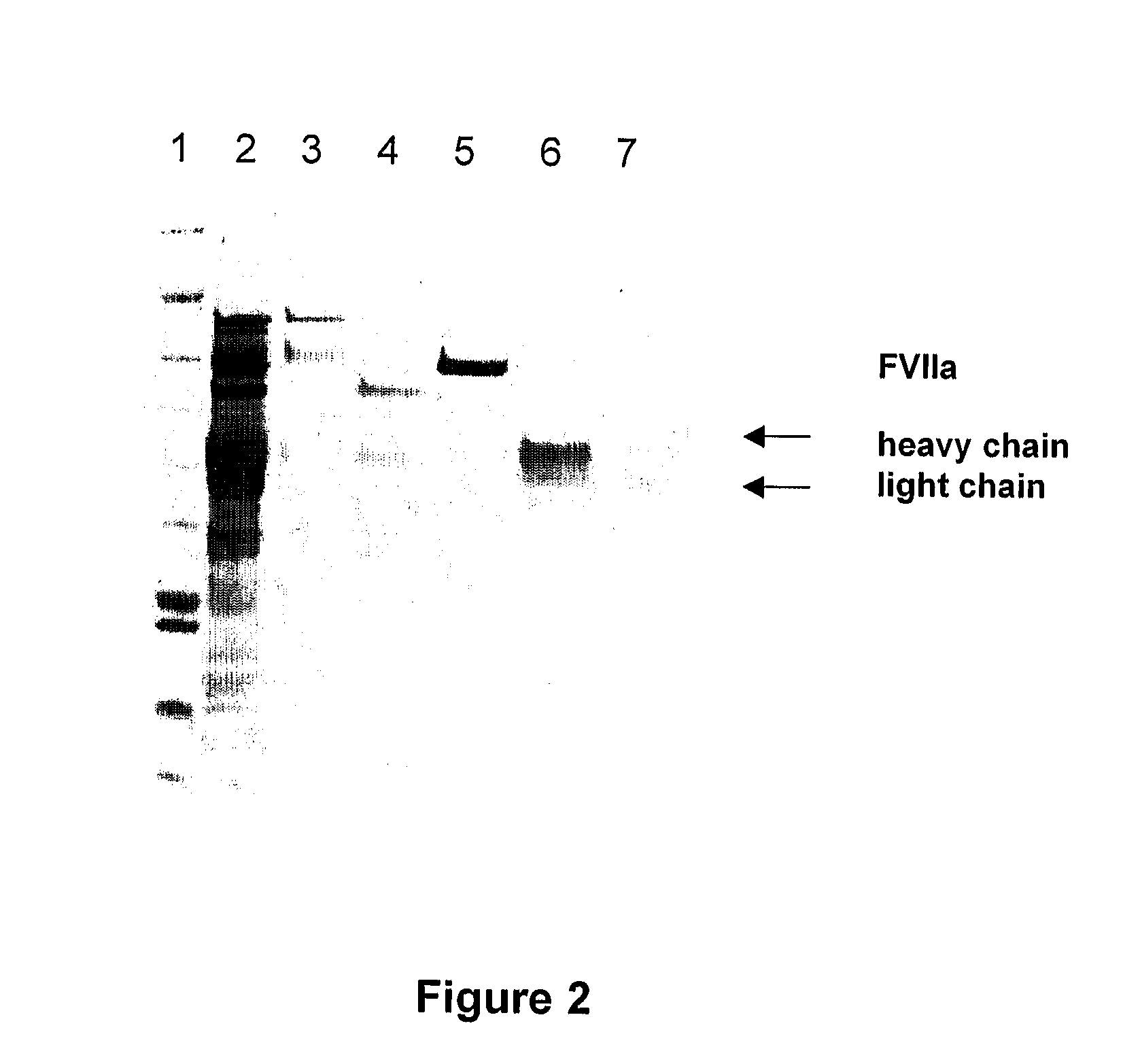


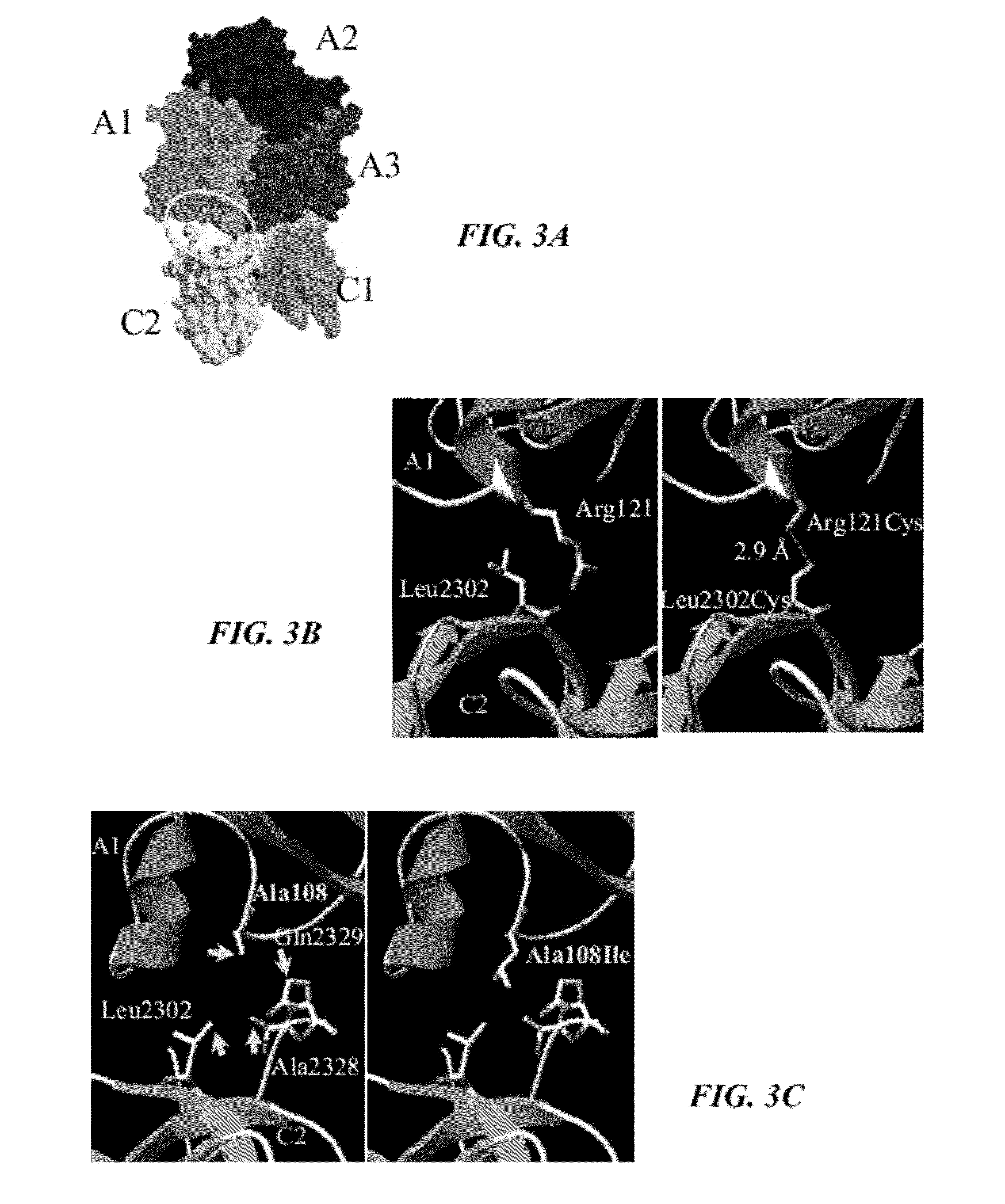

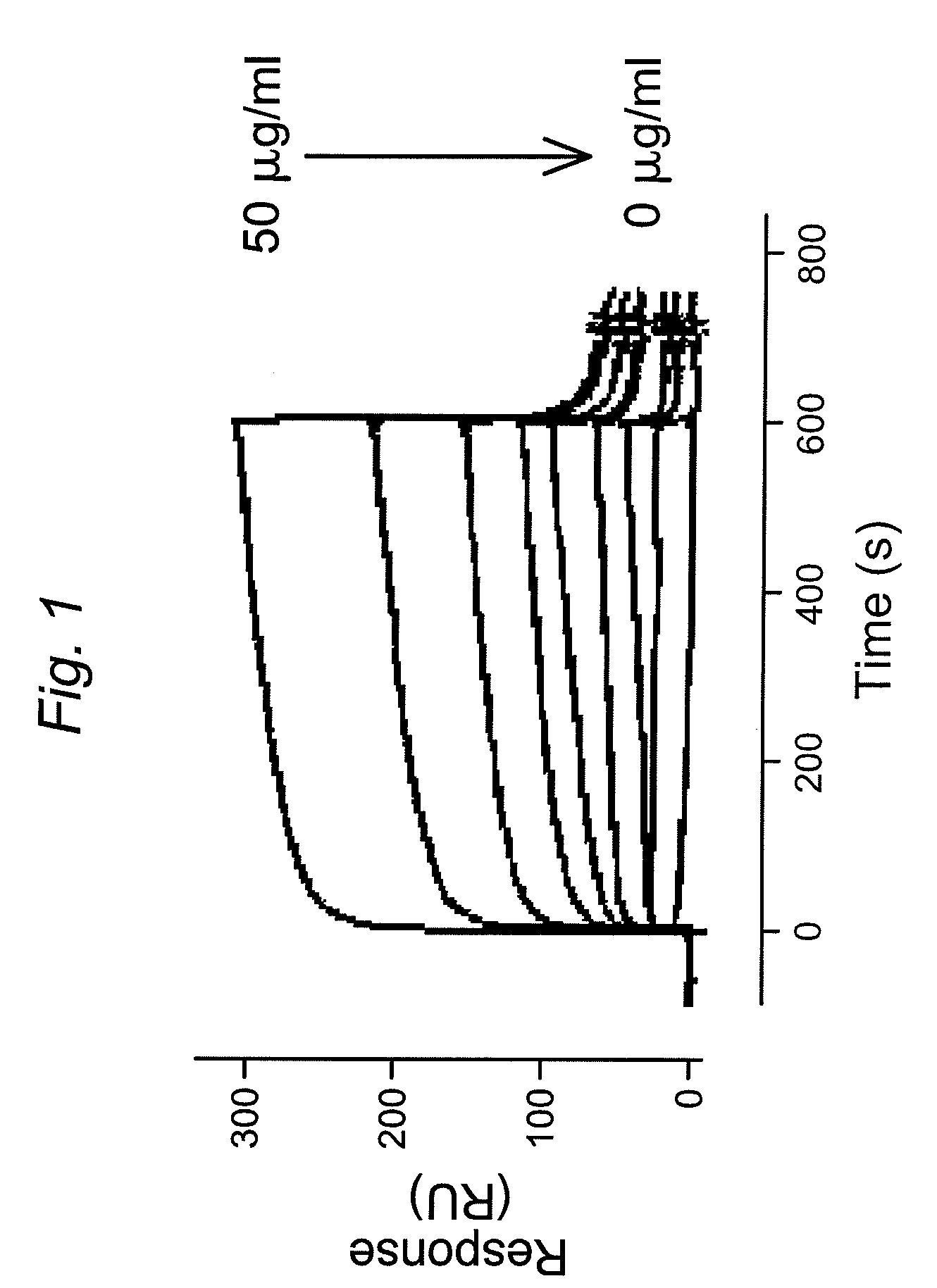
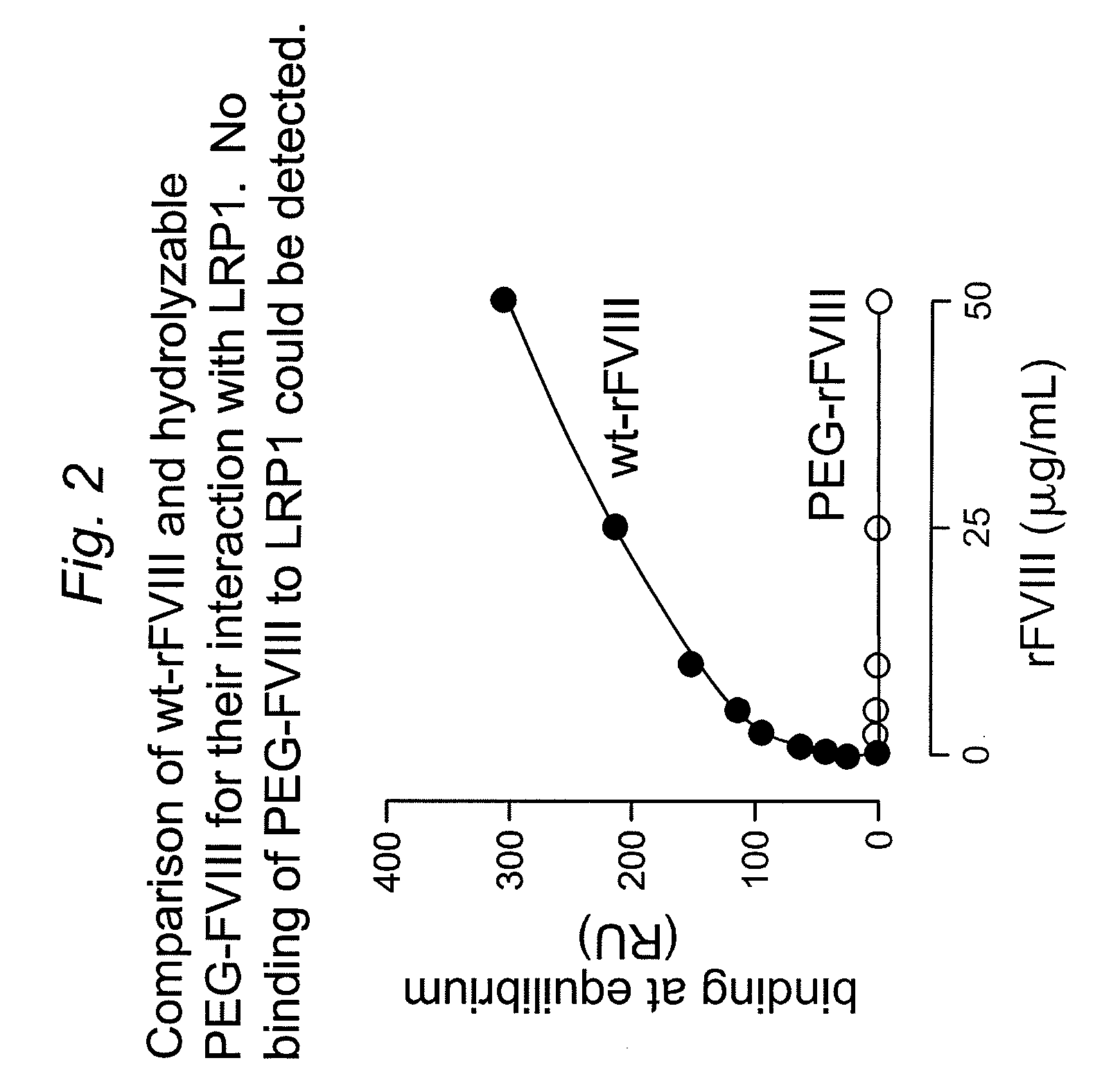
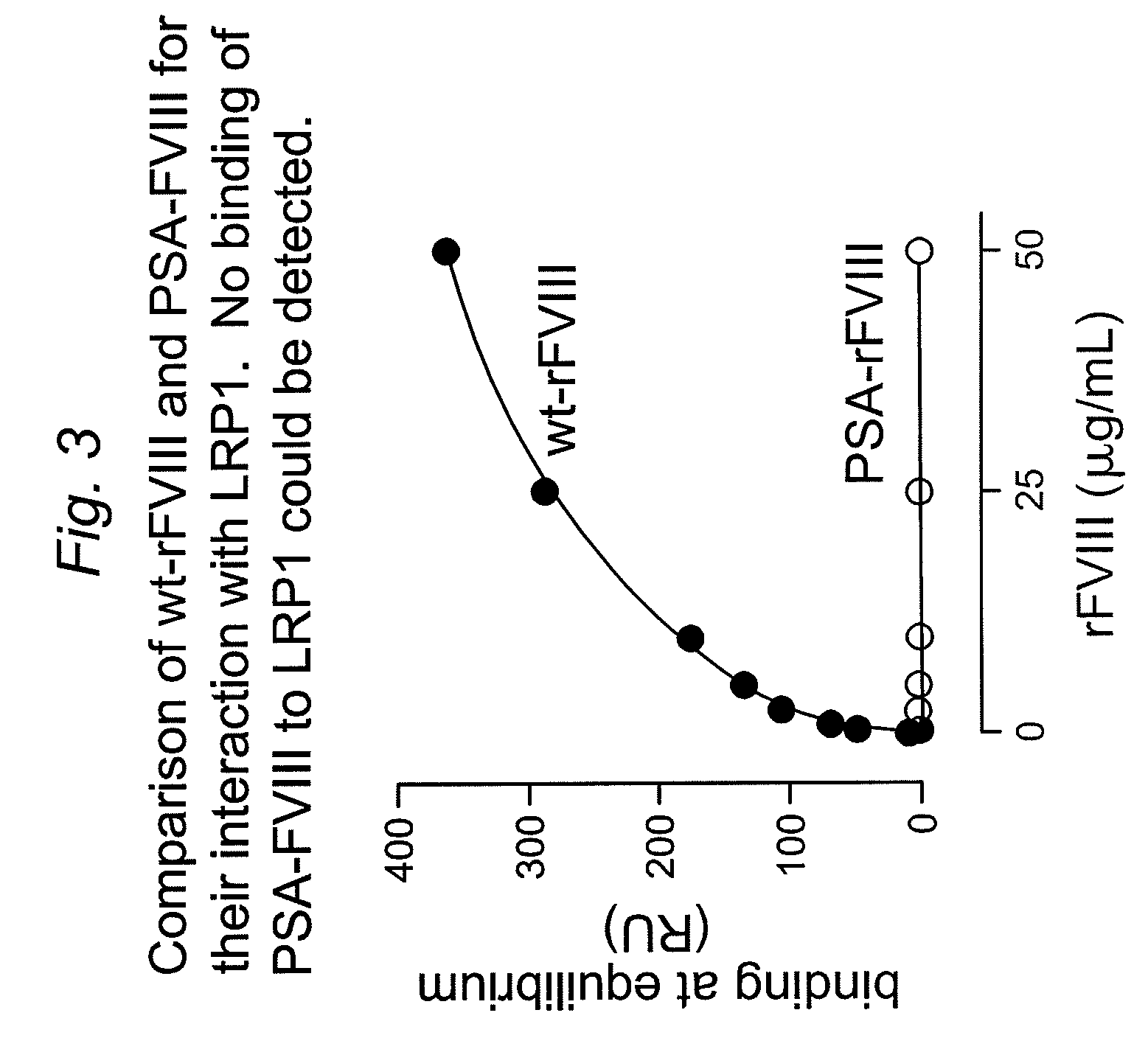




![Demannosylated recombinant factor viii [[vii]] for the treatment of patients with haemophilia a Demannosylated recombinant factor viii [[vii]] for the treatment of patients with haemophilia a](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/de8b7244-99c8-43b2-81e4-d70e4ac6493c/US20100197578A1-20100805-D00001.png)
![Demannosylated recombinant factor viii [[vii]] for the treatment of patients with haemophilia a Demannosylated recombinant factor viii [[vii]] for the treatment of patients with haemophilia a](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/de8b7244-99c8-43b2-81e4-d70e4ac6493c/US20100197578A1-20100805-D00002.png)
![Demannosylated recombinant factor viii [[vii]] for the treatment of patients with haemophilia a Demannosylated recombinant factor viii [[vii]] for the treatment of patients with haemophilia a](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/de8b7244-99c8-43b2-81e4-d70e4ac6493c/US20100197578A1-20100805-D00003.png)