De-immunized factor viii molecule and pharmaceutical compositions comprising the same
A factor, recombinant factor technology, applied in the field of therapeutic proteins, can solve the problems of unsuccessful ITI
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
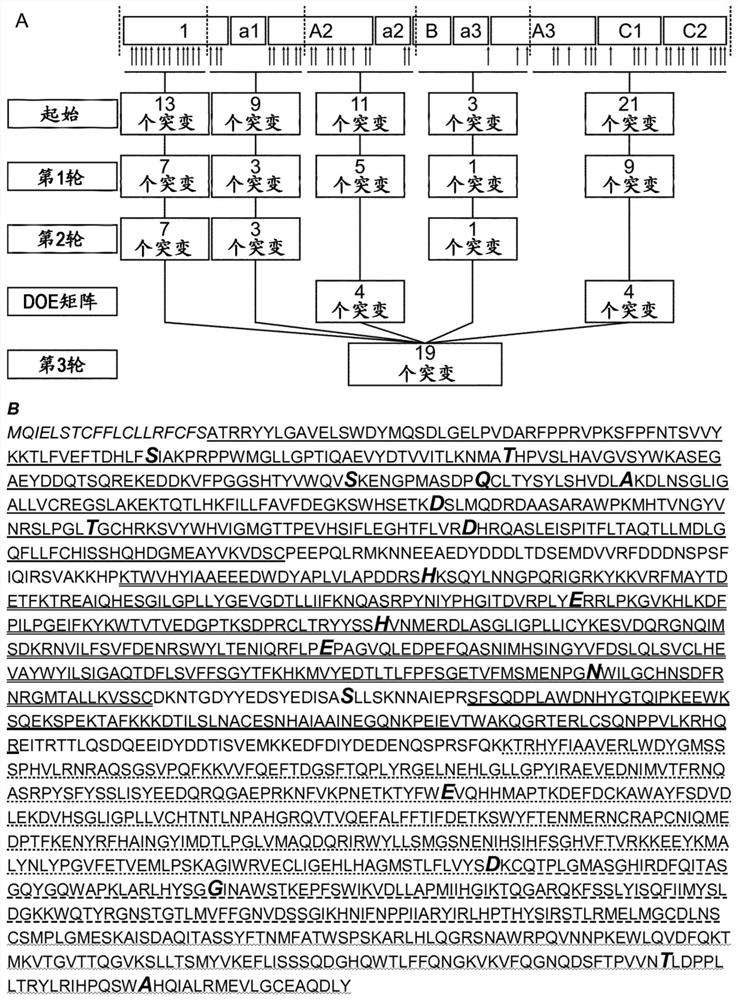
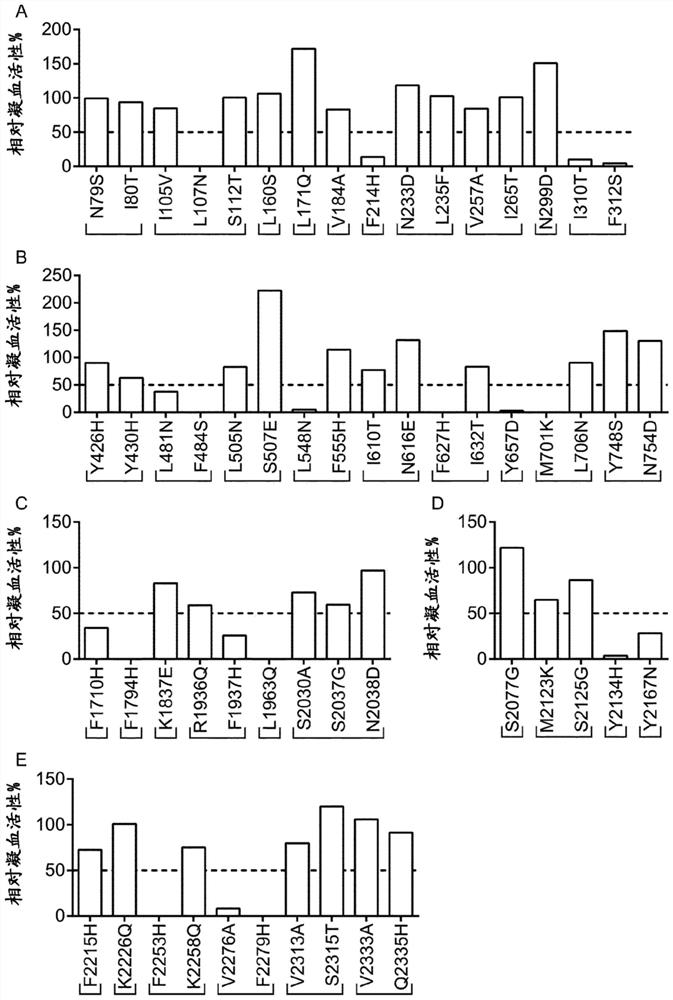
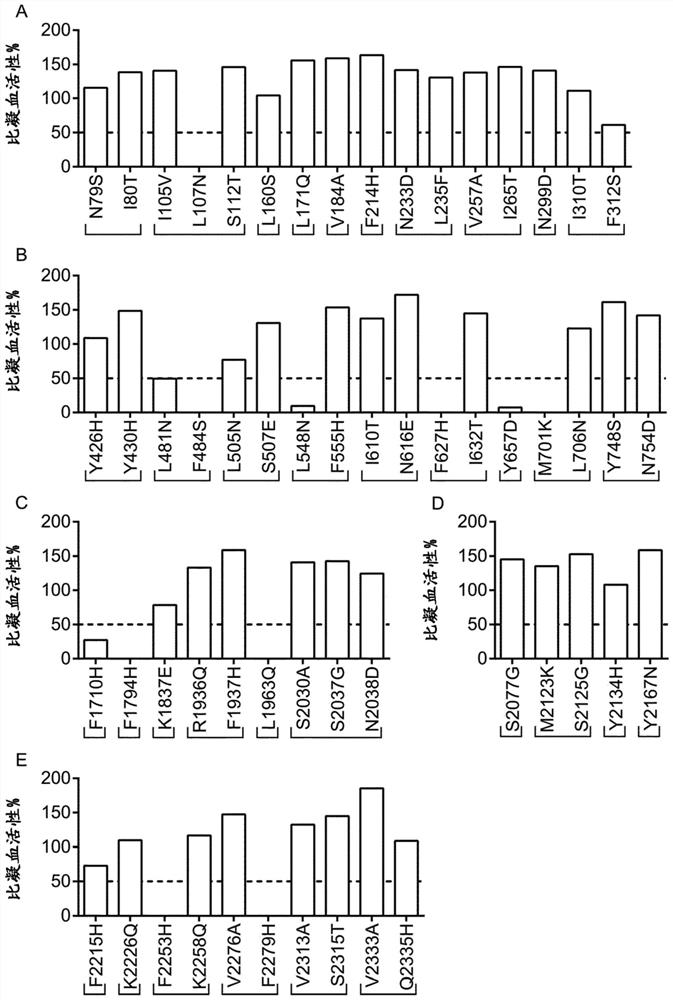
[0242] Using EpiMatrix tools (Epivax, Providence, RI, USA) and the ClustiMer algorithm to conduct a preliminary computer analysis of peptides that bind to MHC class II (T cell epitopes) in human FVIII, EpiMatrix tools can predict eight common class II supertypes, etc. Binding potential of alleles (DRB1*0101, DRB1*0301, DRB1*0401, DRB1*0701, DRB1*0801, DRB1*1110, DRB1*1301, DRB1*1501), which cover most of the population (>90%) , the ClustiMer algorithm identifies clusters of putative T-cell epitopes (assigned immunogenic clusters). The FVIII sequence (FVIII-6rs) shown in SEQ ID NO: 2 was analyzed, which contained 1514 amino acids, excluding the signal sequence of 19 amino acids and the B domain of 818 amino acids. Amino acids of the excluded B domain do not interfere with the furin or thrombin cleavage sites. The computer tool revealed a total of 52 clusters of immunogenic peptides, with cluster scores ranging from 4 to 34, indicating high affinity at high values and lower a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com