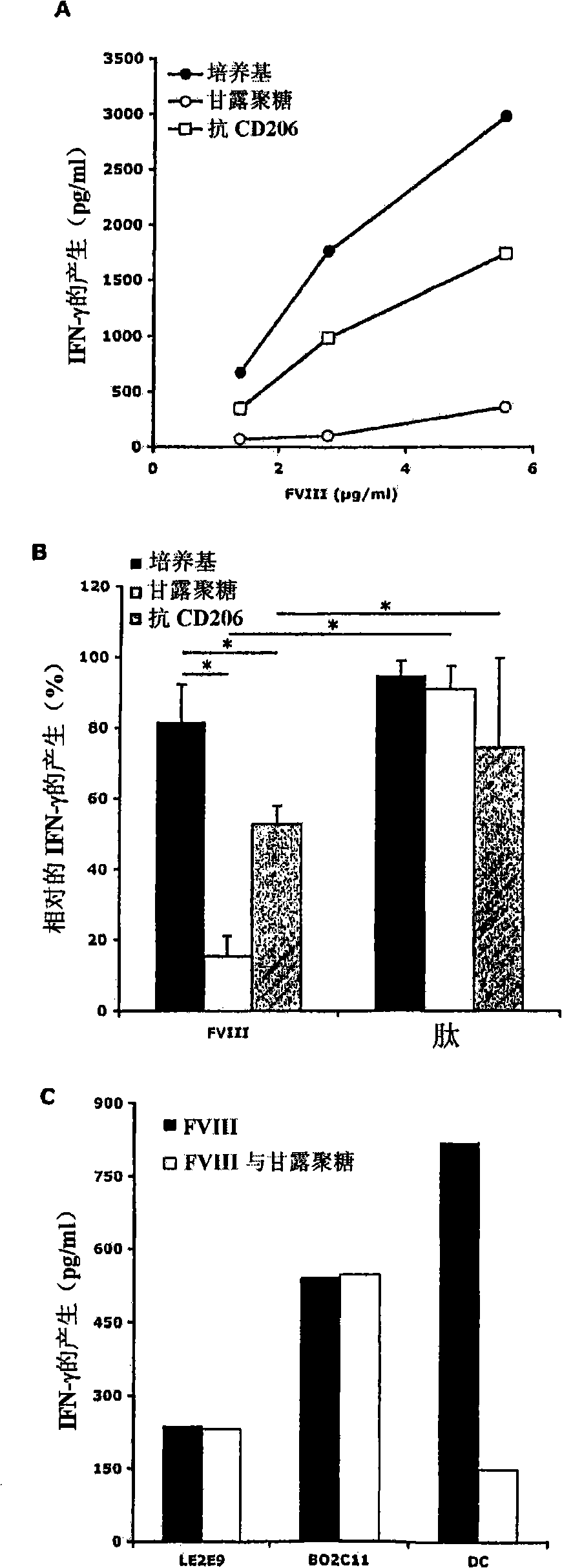
Demannosylated recombinant factor viii for the treatment of patients with hemophiila a
一种去糖基化、氨基酸的技术,应用在凝血/纤溶因子、血液疾病、基因工程等方向,能够解决增加发病率和死亡率、影响患者生活质量、治疗成本增加等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0408] Example 1: Human DCs derived from monocytes
[0409] Peripheral blood was isolated from heparinized buffy coats of healthy adult donors by adhering to plastic cell culture dishes in RPMI1640 medium supplemented with 10% human AB serum, glutamine, and antibiotics for 60 minutes monocytes. Wash gently 3 times with culture medium to remove non-adherent cells. Adherent monocytes were treated in the presence of 500 IU / ml recombinant human interleukin 4 (rhIL-4) (R&D Systems (Lille, France)) supplemented with 1% human AB serum, antibiotics and 1000 IU / ml recombinant human granulocyte- Macrophage colony-stimulating factor (rhGM-CSF) (Immuno Tools (Friesoythe, Germany)) culture medium. Half of the medium, including all supplements, was changed every 2 days. After 5 days of culture, non-adherent cells and loosely attached cells (corresponding to the DC-enriched fraction) were harvested, washed and used for subsequent experiments.
Embodiment 2
[0410] Example 2: Binding of human recombinant full-length FVIII, human recombinant FVIII with B domain deletion to fluorescein
[0411] Human recombinant full-length FVIII (1000IU, Kogenate, Bayer), recombinant human FVIII (BDD-FVIII, 1000IU, Refacto Wyeth) dissolved in water, and containing 5mM CaCl 2 Sodium bicarbonate buffer (pH 9.2) was dialyzed at 4°C, and then coupled with fluorescein-5-isothiocyanate (isomer I, Sigma-Aldrich, Saint Quentin Fallavier, France) at 4°C 7-8 hours. The labeled FVIII was further dialyzed against RPMI1640 medium to remove unconjugated FITC. FVIII-FITC was quantified by Bradford assay using bovine serum albumin as standard.
Embodiment 3
[0412] Example 3: Properties of receptors involved in FVIII endocytosis
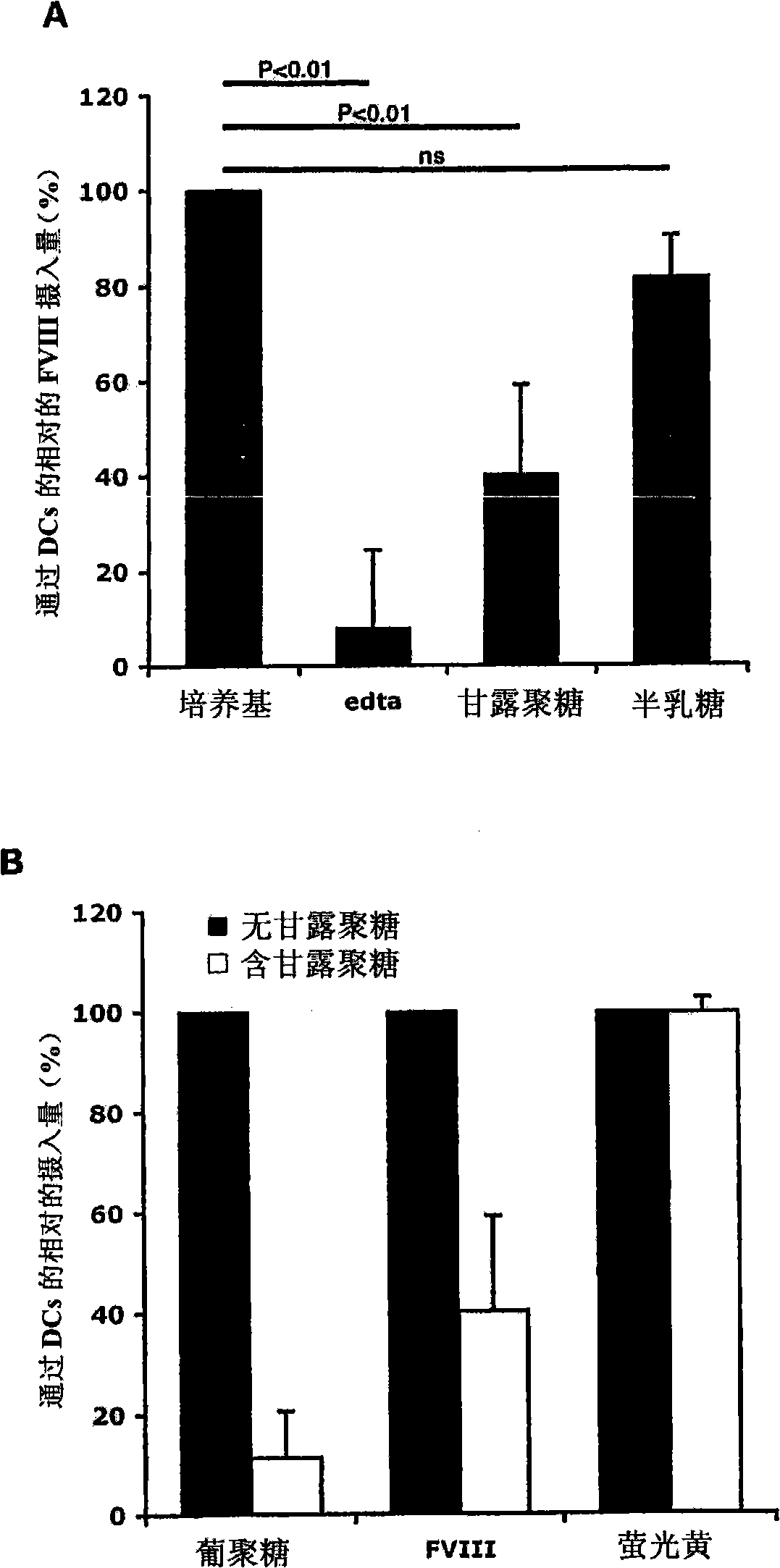
[0413] DCs were incubated with 5 mM EDTA, mannan (1 mg / ml) or galactose (1 mg / ml) at 37°C for 30 minutes, and then added with FVIII-FITC (40 μg / ml) for 2 hours. Endocytosis at 4°C served as a control (not shown).
[0414] result
[0415] figure 1 A : In the presence of EDTA, the endocytosis of FVIII-FITC was inhibited by 92±16.5% (P<0.01). This data suggests a role for divalent ion-dependent receptors in the process of FVIII endocytosis by DCs. The polysaccharide mannan (competitive ligand for mannose-sensitive uptake) reduced FVIII-FITC intake by 60±19% (P<0.01), while galactose (competitive ligand for galactose-sensitive uptake) body) had no significant effect.
[0416] figure 1 B : In our experiments, the specificity of mannan for mannose-sensitive CLRs was determined by using FITC-labeled dextran (a typical ligand for mannose-sensitive CLRs (especially CD206)) and fluorescent Yellow (LY),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com